Insulin-like growth factor binding protein 2 is a growth inhibitory protein conserved in zebrafish
- PMID: 10611375
- PMCID: PMC24810
- DOI: 10.1073/pnas.96.26.15274
Insulin-like growth factor binding protein 2 is a growth inhibitory protein conserved in zebrafish
Abstract
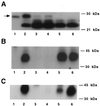
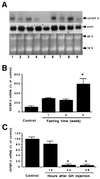
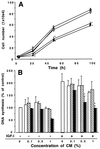
Fish serum contains several specific binding proteins for insulin-like growth factors (IGFBPs). The structure and physiological function of these fish IGFBPs are unknown. Here we report the complete primary sequence of a zebrafish IGFBP deduced from cDNA clones isolated by library screening and rapid amplification of cDNA ends. The full-length 1,757-bp cDNA encodes a protein of 276 aa, which contains a putative 22-residue signal peptide and a 254-residue mature protein. The mature zebrafish IGFBP has a predicted molecular size of 28,440 Da and shows high sequence identity with human IGFBP-2 (52%). The sequence identities with other human IGFBPs are <37%. Chinese hamster ovary cells stably transfected with the zebrafish IGFBP-2 cDNA secreted a 31-kDa protein, which bound to IGF-I and IGF-II with high affinity, but did not bind to Des(1-3)IGF-I or insulin. Northern blot analyses revealed that the zebrafish IGFBP-2 transcript is a 1.8-kb band expressed in many embryonic and adult tissues. In adult zebrafish, IGFBP-2 mRNA levels were greatly reduced by growth hormone treatment but increased by prolonged fasting. When overexpressed or added to cultured zebrafish and mammalian cells, the zebrafish IGFBP-2 significantly inhibited IGF-I-stimulated cell proliferation and DNA synthesis. These results indicate that zebrafish IGFBP-2 is a negative growth regulator acting downstream in the growth hormone-IGF-I axis.
Figures


References
-
- Cock A G. Q Rev Biol. 1966;41:131–190. - PubMed
-
- Siharath K, Bern H A. Proc Zool Soc (Calcutta) 1993;3:113–124.
-
- Dickhoff W W, Beckman B R, Larsen D A, Duan C, Moriyama S. Fish Physiol Biochem. 1997;17:231–236.
-
- Plisetskaya E M. Comp Biochem Physiol B. 1998;121:3–11. - PubMed
-
- Duan C. J Nutr. 1998;128:306S–314S. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Molecular Biology Databases
