The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter
- PMID: 10613863
- PMCID: PMC94240
- DOI: 10.1128/JB.182.1.57-66.2000
The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter
Erratum in
- J Bacteriol 2000 May;182(10):2992
Abstract
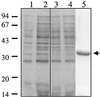
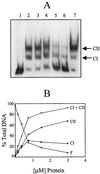
Regulation of toxin production in the gram-positive anaerobe Clostridium perfringens occurs at the level of transcription and involves a two-component signal transduction system. The sensor histidine kinase is encoded by the virS gene, while its cognate response regulator is encoded by the virR gene. We have constructed a VirR expression plasmid in Escherichia coli and purified the resultant His-tagged VirR protein. Gel mobility shift assays demonstrated that VirR binds to the region upstream of the pfoA gene, which encodes perfringolysin O, but not to regions located upstream of the VirR-regulated plc, colA, and pfoR genes, which encode alpha-toxin, collagenase, and a putative pfoA regulator, respectively. The VirR binding site was shown by DNase I footprinting to be a 52-bp core sequence situated immediately upstream of the pfoA promoter. When this region was deleted, VirR was no longer able to bind to the pfoA promoter. The binding site was further localized to two imperfect direct repeats (CCCAGTTNTNCAC) by site-directed mutagenesis. Binding and protection analysis of these mutants indicated that VirR had the ability to bind independently to the two repeated sequences. Based on these observations it is postulated that the VirR positively regulates the synthesis of perfringolysin O by binding directly to a region located immediately upstream of the pfoA promoter and activating transcription.
Figures






References
-
- Albright L M, Huala E, Ausubel F M. Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet. 1989;23:311–336. - PubMed
-
- Awad M M, Bryant A E, Stevens D L, Rood J I. Virulence studies on chromosomal α-toxin and θ-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of α-toxin in Clostridium perfringens-mediated gas gangrene. Mol Microbiol. 1995;15:191–202. - PubMed
-
- Bellsolell L, Prieto J, Serrano L, Coll M. Magnesium binding to the bacterial chemotaxis protein CheY results in large conformational changes involving its functional surface. J Mol Biol. 1994;238:489–495. - PubMed
-
- Boucher P E, Menozzi F D, Locht C. The modular architecture of bacterial response regulators. Insights into the activation mechanism of the BvgA transactivator of Bordetella pertussis. J Mol Biol. 1994;241:363–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

