Copper/zinc-Superoxide dismutase is required for oxytetracycline resistance of Saccharomyces cerevisiae
- PMID: 10613865
- PMCID: PMC94242
- DOI: 10.1128/JB.182.1.76-80.2000
Copper/zinc-Superoxide dismutase is required for oxytetracycline resistance of Saccharomyces cerevisiae
Abstract
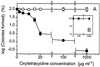
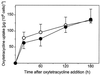
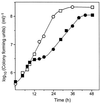
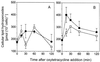
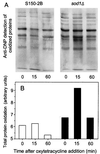
Saccharomyces cerevisiae, along with other eukaryotes, is resistant to tetracyclines. We found that deletion of SOD1 (encoding Cu/Zn superoxide dismutase) rendered S. cerevisiae hypersensitive to oxytetracycline (OTC): a sod1Delta mutant exhibited a >95% reduction in colony-forming ability at an OTC concentration of 20 microg ml(-1), whereas concentrations of up to 1,000 microg ml(-1) had no effect on the growth of the wild type. OTC resistance was restored in the sod1Delta mutant by complementation with wild-type SOD1. The effect of OTC appeared to be cytotoxic and was not evident in a ctt1Delta (cytosolic catalase) mutant or in the presence of tetracycline. SOD1 transcription was not induced by OTC, suggesting that constitutive SOD1 expression is sufficient for wild-type OTC resistance. OTC uptake levels in wild-type and sod1Delta strains were similar. However, lipid peroxidation and protein oxidation were both enhanced during exposure of the sod1Delta mutant, but not the wild type, to OTC. We propose that Sod1p protects S. cerevisiae against a mode of OTC action that is dependent on oxidative damage.
Figures
References
-
- Abdullahi S U, Adeyanju J B. Adverse reaction to oxytetracycline in a dog. Vet Human Toxicol. 1985;27:390–395. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1999.
-
- Chang E C, Kosman D J. Intracellular Mn(II)-associated superoxide scavenging activity protects Cu,Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J Biol Chem. 1989;264:12172–12178. - PubMed
-
- Cormack B P, Bertram G, Egerton M, Gow N A R, Falkow S, Brown A J P. Yeast-enhanced green fluorescent protein (yEGFP): a reporter of gene expression in Candida albicans. Microbiology. 1997;143:303–311. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

