Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis
- PMID: 10613907
- PMCID: PMC2174250
- DOI: 10.1083/jcb.147.7.1493
Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis
Abstract
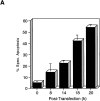
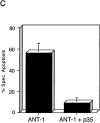
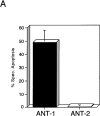
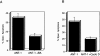
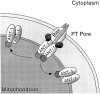
Here, we describe the isolation of adenine nucleotide translocase-1 (ANT-1) in a screen for dominant, apoptosis-inducing genes. ANT-1 is a component of the mitochondrial permeability transition complex, a protein aggregate connecting the inner with the outer mitochondrial membrane that has recently been implicated in apoptosis. ANT-1 expression led to all features of apoptosis, such as phenotypic alterations, collapse of the mitochondrial membrane potential, cytochrome c release, caspase activation, and DNA degradation. Both point mutations that impair ANT-1 in its known activity to transport ADP and ATP as well as the NH(2)-terminal half of the protein could still induce apoptosis. Interestingly, ANT-2, a highly homologous protein could not lead to cell death, demonstrating the specificity of the signal for apoptosis induction. In contrast to Bax, a proapoptotic Bcl-2 gene, ANT-1 was unable to elicit a form of cell death in yeast. This and the observed repression of apoptosis by the ANT-1-interacting protein cyclophilin D suggest that the suicidal effect of ANT-1 is mediated by specific protein-protein interactions within the permeability transition pore.
Figures











References
-
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith A., Struhl K. Current Protocols in Molecular Biology. Wiley Interscience; New York: 1991.
-
- Brustovetsky N., Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ . Biochemistry. 1996;35:8483–8488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

