Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium
- PMID: 10613910
- PMCID: PMC2174247
- DOI: 10.1083/jcb.147.7.1533
Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium
Abstract
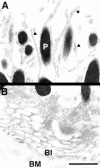
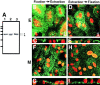
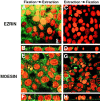
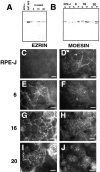
Ezrin, a member of the ezrin/radixin/moesin (ERM) family, localizes to microvilli of epithelia in vivo, where it bridges actin filaments and plasma membrane proteins. Here, we demonstrate two specific morphogenetic roles of ezrin in the retinal pigment epithelium (RPE), i.e., the formation of very long apical microvilli and of elaborate basal infoldings typical of these cells, and characterize the role of ezrin in these processes using antisense and transfection approaches. In the adult rat RPE, only ezrin (no moesin or radixin) was detected at high levels by immunofluorescence and immunoelectron microscopy at microvilli and basal infoldings. At the time when these morphological differentiations develop, in the first two weeks after birth, ezrin levels increased fourfold to adult levels. Addition of ezrin antisense oligonucleotides to primary cultures of rat RPE drastically decreased both apical microvilli and basal infoldings. Transfection of ezrin cDNA into the RPE-J cell line, which has only trace amounts of ezrin and moesin, sparse and stubby apical microvilli, and no basal infoldings, induced maturation of microvilli and the formation of basal infoldings without changing moesin expression levels. Taken together, the results indicate that ezrin is a major determinant in the maturation of surface differentiations of RPE independently of other ERM family members.
Figures






References
-
- Amieva M.R., Litman P., Huang L., Ichimaru E., Furthmayr H. Disruption of dynamic cell surface architecture of NIH3T3 fibroblasts by the N-terminal domains of moesin and ezrinin vivo imaging with GFP fusion proteins. J. Cell Sci. 1998;112:111–125. - PubMed
-
- Andreoli C., Martin M., Le Borgne R., Reggio H., Mangeat P. Ezrin has properties to self-associate at the plasma membrane. J. Cell Sci. 1994;107:2509–2521. - PubMed
-
- Arpin M., Algrain M., Louvard D. Membrane-actin microfilament connectionsan increasing diversity of players related to band 4.1. Curr. Opin. Cell Biol. 1994;6:136–141. - PubMed
-
- Barila D., Murgia C., Nobili F., Perozzi G. Transcriptional regulation of the ezrin gene during rat intestinal development and epithelial differentiation. Biochim. Biophys. Acta. 1995;1263:133–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

