Neutralization assay for human group C rotaviruses using a reverse passive hemagglutination test for endpoint determination
- PMID: 10618062
- PMCID: PMC86016
- DOI: 10.1128/JCM.38.1.50-54.2000
Neutralization assay for human group C rotaviruses using a reverse passive hemagglutination test for endpoint determination
Abstract
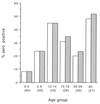
A novel neutralization assay for human group C rotavirus (CHRV) was developed by using a reverse passive hemagglutination (RPHA) test for endpoint determination. In this assay, the neutralization (N)-RPHA test, serial twofold dilutions of sera were mixed with a solution of CHRV that yielded an RPHA test titer of 8 at 3 days after infection. The mixtures were incubated at 37 degrees C for 1 h and were inoculated onto CaCo-2 cell monolayers in a 96-well microplate. Maintenance medium containing 100 microgram of pancreatin per ml was placed in each well. The plate was sealed with sticky plastic film and was incubated at 37 degrees C for 3 days under continuous rotation. Then, the RPHA test titer of each well was determined. The neutralization titer was expressed as the reciprocal of the maximum dilution of the serum that exhibited a fourfold (75%) or greater reduction in the RPHA test titer (8 to 2 or less). Seroconversion of neutralizing antibody was demonstrated by this method in four sets of paired serum specimens from patients with diarrheal disease caused by CHRV. The seroprevalence of CHRV in the general population in Okayama Prefecture was 26.8% by immunofluorescence and 25.5% by the N-RPHA test. The N-RPHA test described here is the first system used to assay for a neutralization antibody against CHRV and is applicable in both clinical and epidemiological settings.
Figures
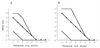
References
-
- Bernstein D I, Kacica M A, McNeal M M, Schiff G M, Ward R L. Local and systemic antibody response to rotavirus WC3 vaccine in adult volunteers. Antivir Res. 1989;12:293–300. - PubMed
-
- Bonsdorf C H V, Svensson L. Human serogroup C rotavirus in Finland. Scand J Infect Dis. 1988;20:475–478. - PubMed
-
- Caul E O, Ashley C R, Darville J M, Bridger J C. Group C rotavirus associated with fatal enteritis in a family outbreak. J Med Virol. 1990;30:201–205. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

