Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice
- PMID: 10618120
- PMCID: PMC88728
- DOI: 10.1128/JCM.38.1.382-388.2000
Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice
Abstract
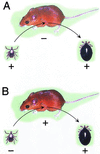
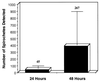
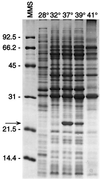
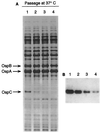
The Lyme disease-associated spirochete, Borrelia burgdorferi, is maintained in enzootic cycles involving Ixodes ticks and small mammals. Previous studies demonstrated that B. burgdorferi expresses outer surface protein A (OspA) but not OspC when residing in the midgut of unfed ticks. However, after ticks feed on blood, some spirochetes stop making OspA and express OspC. Our current work examined the timing and frequency of OspA and OspC expression by B. burgdorferi in infected Ixodes scapularis nymphs as they fed on uninfected mice and in uninfected I. scapularis larvae and nymphs as they first acquired spirochetes from infected mice. Smears of midguts from previously infected ticks were prepared at 12- or 24-h intervals following attachment through repletion at 96 h, and spirochetes were stained for immunofluorescence for detection of antibodies to OspA and OspC. As shown previously, prior to feeding spirochetes in nymphs expressed OspA but not OspC. During nymphal feeding, however, the proportion of spirochetes expressing OspA decreased, while spirochetes expressing OspC became detectable. In fact, spirochetes rapidly began to express OspC, with the greatest proportion of spirochetes having this protein at 48 h of attachment and then with the proportion decreasing significantly by the time that the ticks had completed feeding. In vitro cultivation of the spirochete at different temperatures showed OspC to be most abundant when the spirochetes were grown at 37 degrees C. Yet, the synthesis of this protein waned with continuous passage at this temperature. Immunofluorescence staining of spirochetes in smears of midguts from larvae and nymphs still attached or having completed feeding on infected mice demonstrated that OspA but not OspC was produced by these spirochetes recently acquired from mice. Therefore, the temporal synthesis of OspC by spirochetes only in feeding ticks that were infected prior to the blood meal suggests that this surface protein is involved in transmission from tick to mammal but not from mammal to tick.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

