Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. strain PCC 6803
- PMID: 10618204
- PMCID: PMC91786
- DOI: 10.1128/AEM.66.1.64-72.2000
Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. strain PCC 6803
Abstract
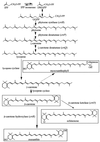
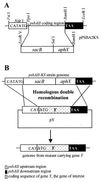
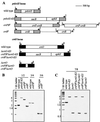
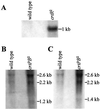
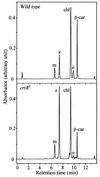
The psbAII locus was used as an integration platform to overexpress genes involved in carotenoid biosynthesis in Synechocystis sp. strain PCC 6803 under the control of the strong psbAII promoter. The sequences of the genes encoding the yeast isopentenyl diphosphate isomerase (ipi) and the Synechocystis beta-carotene hydroxylase (crtR) and the linked Synechocystis genes coding for phytoene desaturase and phytoene synthase (crtP and crtB, respectively) were introduced into Synechocystis, replacing the psbAII coding sequence. Expression of ipi, crtR, and crtP and crtB led to a large increase in the corresponding transcript levels in the mutant strains, showing that the psbAII promoter can be used to drive transcription and to overexpress various genes in Synechocystis. Overexpression of crtP and crtB led to a 50% increase in the myxoxanthophyll and zeaxanthin contents in the mutant strain, whereas the beta-carotene and echinenone contents remained unchanged. Overexpression of crtR induced a 2.5-fold increase in zeaxanthin accumulation in the corresponding overexpressing mutant compared to that in the wild-type strain. In this mutant strain, zeaxanthin becomes the major pigment (more than half the total amount of carotenoid) and the beta-carotene and echinenone amounts are reduced by a factor of 2. However, overexpression of ipi did not result in a change in the carotenoid content of the mutant. To further alter the carotenoid content of Synechocystis, the crtO gene, encoding beta-carotene ketolase, which converts beta-carotene to echinenone, was disrupted in the wild type and in the overexpressing strains so that they no longer produced echinenone. In this way, by a combination of overexpression and deletion of particular genes, the carotenoid content of cyanobacteria can be altered significantly.
Figures

Similar articles
-
The zeaxanthin biosynthesis enzyme beta-carotene hydroxylase is involved in myxoxanthophyll synthesis in Synechocystis sp. PCC 6803.FEBS Lett. 1999 Jul 9;454(3):247-51. doi: 10.1016/s0014-5793(99)00817-0. FEBS Lett. 1999. PMID: 10431816
-
Substrate specificities and availability of fucosyltransferase and beta-carotene hydroxylase for myxol 2'-fucoside synthesis in Anabaena sp. strain PCC 7120 compared with Synechocystis sp. strain PCC 6803.J Bacteriol. 2008 Oct;190(20):6726-33. doi: 10.1128/JB.01881-07. Epub 2008 Aug 15. J Bacteriol. 2008. PMID: 18708496 Free PMC article.
-
Characterization of cyanobacterial carotenoid ketolase CrtW and hydroxylase CrtR by complementation analysis in Escherichia coli.Plant Cell Physiol. 2008 Dec;49(12):1867-78. doi: 10.1093/pcp/pcn169. Epub 2008 Nov 5. Plant Cell Physiol. 2008. PMID: 18987067
-
Carotenoids, versatile components of oxygenic photosynthesis.Prog Lipid Res. 2013 Oct;52(4):539-61. doi: 10.1016/j.plipres.2013.07.001. Epub 2013 Jul 26. Prog Lipid Res. 2013. PMID: 23896007 Review.
-
Metabolic engineering of the astaxanthin-biosynthetic pathway of Xanthophyllomyces dendrorhous.FEMS Yeast Res. 2003 Dec;4(3):221-31. doi: 10.1016/S1567-1356(03)00158-2. FEMS Yeast Res. 2003. PMID: 14654426 Review.
Cited by
-
Bio-solar cell factories for photosynthetic isoprenoids production.Planta. 2019 Jan;249(1):181-193. doi: 10.1007/s00425-018-2969-8. Epub 2018 Aug 4. Planta. 2019. PMID: 30078076 Review.
-
Rhythm of the Night (and Day): Predictive Metabolic Modeling of Diurnal Growth in Chlamydomonas.mSystems. 2022 Aug 30;7(4):e0017622. doi: 10.1128/msystems.00176-22. Epub 2022 Jun 13. mSystems. 2022. PMID: 35695419 Free PMC article.
-
A photoactive carotenoid protein acting as light intensity sensor.Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):12075-80. doi: 10.1073/pnas.0804636105. Epub 2008 Aug 7. Proc Natl Acad Sci U S A. 2008. PMID: 18687902 Free PMC article.
-
Functional analysis of photosynthetic pigment binding complexes in the green alga Haematococcus pluvialis reveals distribution of astaxanthin in Photosystems.Sci Rep. 2017 Nov 24;7(1):16319. doi: 10.1038/s41598-017-16641-6. Sci Rep. 2017. PMID: 29176710 Free PMC article.
-
Synthetic biology in marine cyanobacteria: Advances and challenges.Front Microbiol. 2022 Sep 16;13:994365. doi: 10.3389/fmicb.2022.994365. eCollection 2022. Front Microbiol. 2022. PMID: 36188008 Free PMC article. Review.
References
-
- Anderson M S, Muehlbacher M, Street I P, Proffitt J, Poulter C D. Isopentenyl diphosphate:dimethylallyl diphosphate isomerase. An improved purification of the enzyme and isolation of the gene from Saccharomyces cerevisiae. J Biol Chem. 1989;264:19169–19175. - PubMed
-
- Armstrong G A. Genetics of eubacterial carotenoid biosynthesis: a colorful tale. Annu Rev Microbiol. 1997;51:629–659. - PubMed
-
- Bernhard K. Synthetic astaxanthin. The route of a carotenoid from research to commercialization. In: Krinski N I, Mathews-Roth M M, Taylor R F, editors. Carotenoids: chemistry and biology. New York, N.Y: Plenum; 1989. pp. 337–364.
-
- Black T A, Cai Y, Wolk C P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol Microbiol. 1993;9:77–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

