Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods
- PMID: 10618206
- PMCID: PMC91788
- DOI: 10.1128/AEM.66.1.80-86.2000
Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods
Abstract
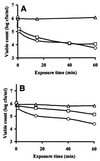
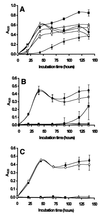
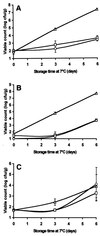
The objective of this study was to determine whether chitosan (poly-beta-1,4-glucosamine) and hydrolysates of chitosan can be used as novel preservatives in foods. Chitosan was hydrolyzed by using oxidative-reductive degradation, crude papaya latex, and lysozyme. Mild hydrolysis of chitosan resulted in improved microbial inactivation in saline and greater inhibition of growth of several spoilage yeasts in laboratory media, but highly degraded products of chitosan exhibited no antimicrobial activity. In pasteurized apple-elderflower juice stored at 7 degrees C, addition of 0.3 g of chitosan per liter eliminated yeasts entirely for the duration of the experiment (13 days), while the total counts and the lactic acid bacterial counts increased at a slower rate than they increased in the control. Addition of 0.3 or 1.0 g of chitosan per kg had no effect on the microbial flora of hummus, a chickpea dip; in the presence of 5.0 g of chitosan per kg, bacterial growth but not yeast growth was substantially reduced compared with growth in control dip stored at 7 degrees C for 6 days. Improved antimicrobial potency of chitosan hydrolysates like that observed in the saline and laboratory medium experiments was not observed in juice and dip experiments. We concluded that native chitosan has potential for use as a preservative in certain types of food but that the increase in antimicrobial activity that occurs following partial hydrolysis is too small to justify the extra processing involved.
Figures




Similar articles
-
The antifungal properties of chitosan in laboratory media and apple juice.Int J Food Microbiol. 1999 Mar 1;47(1-2):67-77. doi: 10.1016/s0168-1605(99)00006-9. Int J Food Microbiol. 1999. PMID: 10357275
-
Antimicrobial characteristics of chitosans against food spoilage microorganisms in liquid media and mayonnaise.Biosci Biotechnol Biochem. 2001 Nov;65(11):2378-83. doi: 10.1271/bbb.65.2378. Biosci Biotechnol Biochem. 2001. PMID: 11791708
-
Antimicrobial action of hydrolyzed chitosan against spoilage yeasts and lactic acid bacteria of fermented vegetables.J Food Prot. 2002 May;65(5):828-33. doi: 10.4315/0362-028x-65.5.828. J Food Prot. 2002. PMID: 12030295
-
Antimicrobial action of exogenous chitosan.EXS. 1999;87:315-33. doi: 10.1007/978-3-0348-8757-1_23. EXS. 1999. PMID: 10906970 Review.
-
Novel natural food antimicrobials.Annu Rev Food Sci Technol. 2012;3:381-403. doi: 10.1146/annurev-food-022811-101241. Annu Rev Food Sci Technol. 2012. PMID: 22385168 Review.
Cited by
-
Bacterial growth on chitosan-coated polypropylene textile.ISRN Microbiol. 2012 Feb 19;2012:749694. doi: 10.5402/2012/749694. Print 2012. ISRN Microbiol. 2012. PMID: 23724330 Free PMC article.
-
Potential of chitosan from Mucor rouxxi UCP064 as alternative natural compound to inhibit Listeria monocytogenes.Braz J Microbiol. 2009 Jul;40(3):583-9. doi: 10.1590/S1517-838220090003000022. Epub 2009 Sep 1. Braz J Microbiol. 2009. PMID: 24031403 Free PMC article.
-
Transcriptional responses of Bacillus cereus towards challenges with the polysaccharide chitosan.PLoS One. 2011;6(9):e24304. doi: 10.1371/journal.pone.0024304. Epub 2011 Sep 8. PLoS One. 2011. PMID: 21931677 Free PMC article.
-
Chitosan-induced antiviral activity and innate immunity in plants.Environ Sci Pollut Res Int. 2015 Feb;22(4):2935-44. doi: 10.1007/s11356-014-3571-7. Epub 2014 Sep 17. Environ Sci Pollut Res Int. 2015. PMID: 25226839
-
Chitosan/Cyclodextrin/TPP Nanoparticles Loaded with Quercetin as Novel Bacterial Quorum Sensing Inhibitors.Molecules. 2017 Nov 15;22(11):1975. doi: 10.3390/molecules22111975. Molecules. 2017. PMID: 29140285 Free PMC article.
References
-
- Ahern T J, Klibanov A M. The mechanism of irreversible enzyme inactivation at 100°C. Science. 1985;228:1280–1284. - PubMed
-
- Allan C R, Hadwiger L A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp Mycol. 1979;3:285–287.
-
- Azarkan M, Amrani A, Nijs M, Vandermeers A, Zerhouni S, Smoulders N, Looze Y. Carica papaya latex is a rich source of class II chitinase. Phytochemistry. 1997;46:1319–1325. - PubMed
-
- Brzezinski R, Boucher I, Dupuy A, Plouffe B. Actinomycetes as model organisms for the study of chitosanases. In: Muzzarelli R A A, Peter M G, editors. Chitin handbook. Grottammare, Italy: European Chitin Society; 1997. pp. 291–296.
-
- Giordani R, Cardenas M L, Moulin-Traffort J, Régli P. Fungicidal activity of latex sap from Carica papaya and antifungal effect of d(+)-glucosamine on Candida albicans growth. Mycoses. 1996;39:103–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

