Evidence for microbial Fe(III) reduction in anoxic, mining-impacted lake sediments (Lake Coeur d'Alene, Idaho)
- PMID: 10618217
- PMCID: PMC91799
- DOI: 10.1128/AEM.66.1.154-162.2000
Evidence for microbial Fe(III) reduction in anoxic, mining-impacted lake sediments (Lake Coeur d'Alene, Idaho)
Abstract
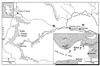
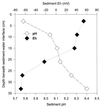
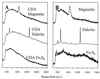
Mining-impacted sediments of Lake Coeur d'Alene, Idaho, contain more than 10% metals on a dry weight basis, approximately 80% of which is iron. Since iron (hydr)oxides adsorb toxic, ore-associated elements, such as arsenic, iron (hydr)oxide reduction may in part control the mobility and bioavailability of these elements. Geochemical and microbiological data were collected to examine the ecological role of dissimilatory Fe(III)-reducing bacteria in this habitat. The concentration of mild-acid-extractable Fe(II) increased with sediment depth up to 50 g kg(-1), suggesting that iron reduction has occurred recently. The maximum concentrations of dissolved Fe(II) in interstitial water (41 mg liter(-1)) occurred 10 to 15 cm beneath the sediment-water interface, suggesting that sulfidogenesis may not be the predominant terminal electron-accepting process in this environment and that dissolved Fe(II) arises from biological reductive dissolution of iron (hydr)oxides. The concentration of sedimentary magnetite (Fe(3)O(4)), a common product of bacterial Fe(III) hydroxide reduction, was as much as 15.5 g kg(-1). Most-probable-number enrichment cultures revealed that the mean density of Fe(III)-reducing bacteria was 8.3 x 10(5) cells g (dry weight) of sediment(-1). Two new strains of dissimilatory Fe(III)-reducing bacteria were isolated from surface sediments. Collectively, the results of this study support the hypothesis that dissimilatory reduction of iron has been and continues to be an important biogeochemical process in the environment examined.
Figures



References
-
- Beard B L, Johnson C M, Cox L, Sun H, Nealson K H, Aguilar C. Iron isotope biosignatures. Science. 1999;285:1889–1892. - PubMed
-
- Belzile N, Tessier A. Interactions between arsenic and iron oxyhydroxides in lacustrine sediments. Geochim Cosmochim Acta. 1990;54:103–109.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

