Exercise-induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: differential effects on insulin-receptor substrates 1 and 2
- PMID: 10618367
- PMCID: PMC26612
- DOI: 10.1073/pnas.97.1.38
Exercise-induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: differential effects on insulin-receptor substrates 1 and 2
Abstract
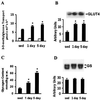
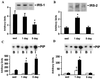
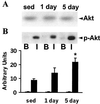
Level of physical activity is linked to improved glucose homeostasis. We determined whether exercise alters the expression and/or activity of proteins involved in insulin-signal transduction in skeletal muscle. Wistar rats swam 6 h per day for 1 or 5 days. Epitrochlearis muscles were excised 16 h after the last exercise bout, and were incubated with or without insulin (120 nM). Insulin-stimulated glucose transport increased 30% and 50% after 1 and 5 days of exercise, respectively. Glycogen content increased 2- and 4-fold after 1 and 5 days of exercise, with no change in glycogen synthase expression. Protein expression of the glucose transporter GLUT4 and the insulin receptor increased 2-fold after 1 day, with no further change after 5 days of exercise. Insulin-stimulated receptor tyrosine phosphorylation increased 2-fold after 5 days of exercise. Insulin-stimulated tyrosine phosphorylation of insulin-receptor substrate (IRS) 1 and associated phosphatidylinositol (PI) 3-kinase activity increased 2.5- and 3. 5-fold after 1 and 5 days of exercise, despite reduced (50%) IRS-1 protein content after 5 days of exercise. After 1 day of exercise, IRS-2 protein expression increased 2.6-fold and basal and insulin-stimulated IRS-2 associated PI 3-kinase activity increased 2. 8-fold and 9-fold, respectively. In contrast to IRS-1, IRS-2 expression and associated PI 3-kinase activity normalized to sedentary levels after 5 days of exercise. Insulin-stimulated Akt phosphorylation increased 5-fold after 5 days of exercise. In conclusion, increased insulin-stimulated glucose transport after exercise is not limited to increased GLUT4 expression. Exercise leads to increased expression and function of several proteins involved in insulin-signal transduction. Furthermore, the differential response of IRS-1 and IRS-2 to exercise suggests that these molecules have specialized, rather than redundant, roles in insulin signaling in skeletal muscle.
Figures
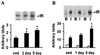
References
-
- Zierath J R. Acta Physiol Scand. 1995;155,(Suppl. 626):1–96. - PubMed
-
- Devlin J T, Hirshman M, Horton E D, Horton E S. Diabetes. 1987;36:434–439. - PubMed
-
- Wallberg-Henriksson H. Acta Physiol Scand. 1987;131,(Suppl. 564):1–80. - PubMed
-
- Wallberg-Henriksson H, Constable S H, Young D A, Holloszy J O. J Appl Physiol. 1988;65:909–913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

