Solid-state synthesis and mechanical unfolding of polymers of T4 lysozyme
- PMID: 10618384
- PMCID: PMC26629
- DOI: 10.1073/pnas.97.1.139
Solid-state synthesis and mechanical unfolding of polymers of T4 lysozyme
Abstract
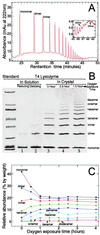
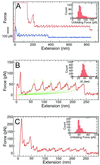
Recent advances in single molecule manipulation methods offer a novel approach to investigating the protein folding problem. These studies usually are done on molecules that are naturally organized as linear arrays of globular domains. To extend these techniques to study proteins that normally exist as monomers, we have developed a method of synthesizing polymers of protein molecules in the solid state. By introducing cysteines at locations where bacteriophage T4 lysozyme molecules contact each other in a crystal and taking advantage of the alignment provided by the lattice, we have obtained polymers of defined polarity up to 25 molecules long that retain enzymatic activity. These polymers then were manipulated mechanically by using a modified scanning force microscope to characterize the force-induced reversible unfolding of the individual lysozyme molecules. This approach should be general and adaptable to many other proteins with known crystal structures. For T4 lysozyme, the force required to unfold the monomers was 64 +/- 16 pN at the pulling speed used. Refolding occurred within 1 sec of relaxation with an efficiency close to 100%. Analysis of the force versus extension curves suggests that the mechanical unfolding transition follows a two-state model. The unfolding forces determined in 1 M guanidine hydrochloride indicate that in these conditions the activation barrier for unfolding is reduced by 2 kcal/mol.
Figures



References
-
- Kellermayer M S, Smith S B, Granzier H L, Bustamante C. Science. 1997;276:1112–1116. - PubMed
-
- Tskhovrebova L, Trinick J, Sleep J A, Simmons R M. Nature (London) 1997;387:308–312. - PubMed
-
- Rief M, Gautel M, Oesterheld F, Fernandez J M, Gaub H E. Science. 1997;276:1109–1112. - PubMed
-
- Oberhauser A F, Marszalek P E, Erickson H P, Fernandez J M. Nature (London) 1998;393:181–185. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

