A human model for multigenic inheritance: phenotypic expression in Hirschsprung disease requires both the RET gene and a new 9q31 locus
- PMID: 10618407
- PMCID: PMC26652
- DOI: 10.1073/pnas.97.1.268
A human model for multigenic inheritance: phenotypic expression in Hirschsprung disease requires both the RET gene and a new 9q31 locus
Abstract
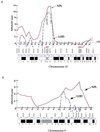
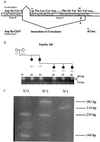
Reduced penetrance in genetic disorders may be either dependent or independent of the genetic background of gene carriers. Hirschsprung disease (HSCR) demonstrates a complex pattern of inheritance with approximately 50% of familial cases being heterozygous for mutations in the receptor tyrosine kinase RET. Even when identified, the penetrance of RET mutations is only 50-70%, gender-dependent, and varies with the extent of aganglionosis. We searched for additional susceptibility genes which, in conjunction with RET, lead to phenotypic expression by studying 12 multiplex HSCR families. Haplotype analysis and extensive mutation screening demonstrated three types of families: six families harboring severe RET mutations (group I); and the six remaining families, five of which are RET-linked families with no sequence alterations and one RET-unlinked family (group II). Although the presence of RET mutations in group I families is sufficient to explain HSCR inheritance, a genome scan reveals a new susceptibility locus on 9q31 exclusively in group II families. As such, the gene at 9q31 is a modifier of HSCR penetrance. These observations imply that identification of new susceptibility factors in a complex disease may depend on classification of families by mutational type at known susceptibility genes.
Figures


References
-
- Chakravarti A, Lander E S. In: Genetics and Biology of Alcoholism. Cloninger C R, Begleiter H, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. pp. 307–315.
-
- Kajiwara K, Berson E L, Dryja T P. Science. 1994;264:1604–1608. - PubMed
-
- Cucca F, Goy J V, Kawaguchi Y, Esposito L, Merriman M E, Wilson A J, Cordell H J, Bain S C, Todd J A. Nat Genet. 1998;19:301–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

