Infusion of alpha-galactosidase A reduces tissue globotriaosylceramide storage in patients with Fabry disease
- PMID: 10618424
- PMCID: PMC26669
- DOI: 10.1073/pnas.97.1.365
Infusion of alpha-galactosidase A reduces tissue globotriaosylceramide storage in patients with Fabry disease
Abstract
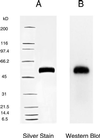
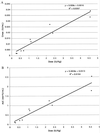
Fabry disease is a lysosomal storage disorder caused by a deficiency of the lysosomal enzyme alpha-galactosidase A (alpha-gal A). This enzymatic defect results in the accumulation of the glycosphingolipid globotriaosylceramide (Gb(3); also referred to as ceramidetrihexoside) throughout the body. To investigate the effects of purified alpha-gal A, 10 patients with Fabry disease received a single i.v. infusion of one of five escalating dose levels of the enzyme. The objectives of this study were: (i) to evaluate the safety of administered alpha-gal A, (ii) to assess the pharmacokinetics of i.v.-administered alpha-gal A in plasma and liver, and (iii) to determine the effect of this replacement enzyme on hepatic, urine sediment and plasma concentrations of Gb(3). alpha-Gal A infusions were well tolerated in all patients. Immunohistochemical staining of liver tissue approximately 2 days after enzyme infusion identified alpha-gal A in several cell types, including sinusoidal endothelial cells, Kupffer cells, and hepatocytes, suggesting diffuse uptake via the mannose 6-phosphate receptor. The tissue half-life in the liver was greater than 24 hr. After the single dose of alpha-gal A, nine of the 10 patients had significantly reduced Gb(3) levels both in the liver and shed renal tubular epithelial cells in the urine sediment. These data demonstrate that single infusions of alpha-gal A prepared from transfected human fibroblasts are both safe and biochemically active in patients with Fabry disease. The degree of substrate reduction seen in the study is potentially clinically significant in view of the fact that Gb(3) burden in Fabry patients increases gradually over decades. Taken together, these results suggest that enzyme replacement is likely to be an effective therapy for patients with this metabolic disorder.
Figures

References
-
- Brady R O, Gal A E, Bradley R M, Martensson E, Warshaw A L, Laster L. N Engl J Med. 1967;276:1163–1167. - PubMed
-
- Desnick R O, Ioannou Y A, Eng C M. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1996. pp. 2741–2784.
-
- Kaye E M, Kolodny E H, Logigian E L, Ullman M D. Ann Neurol. 1988;23:505–509. - PubMed
-
- DeVeber G A, Schwarting G A, Kolodny E H, Kowall N W. Ann Neurol. 1992;31:409–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

