Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein
- PMID: 10618443
- PMCID: PMC26688
- DOI: 10.1073/pnas.97.1.477
Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein
Abstract
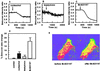
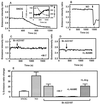
Although the function of metallothionein (MT), a 6- to 7-kDa cysteine-rich metal binding protein, remains unclear, it has been suggested from in vitro studies that MT is an important component of intracellular redox signaling, including being a target for nitric oxide (NO). To directly study the interaction between MT and NO in live cells, we generated a fusion protein consisting of MT sandwiched between two mutant green fluorescent proteins (GFPs). In vitro studies with this chimera (FRET-MT) demonstrate that fluorescent resonance energy transfer (FRET) can be used to follow conformational changes indicative of metal release from MT. Imaging experiments with live endothelial cells show that agents that increase cytoplasmic Ca(2+) act via endogenously generated NO to rapidly and persistently release metal from MT. A role for this interaction in intact tissue is supported by the finding that the myogenic reflex of mesenteric arteries is absent in MT knockout mice (MT(-/-)) unless endogenous NO synthesis is blocked. These results are the first application of intramolecular green fluorescent protein (GFP)-based FRET in a native protein and demonstrate the utility of FRET-MT as an intracellular surrogate indicator of NO production. In addition, an important role of metal thiolate clusters of MT in NO signaling in vascular tissue is revealed.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

