Salmonella typhimurium induces epithelial IL-8 expression via Ca(2+)-mediated activation of the NF-kappaB pathway
- PMID: 10619864
- PMCID: PMC382586
- DOI: 10.1172/JCI8066
Salmonella typhimurium induces epithelial IL-8 expression via Ca(2+)-mediated activation of the NF-kappaB pathway
Abstract
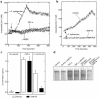
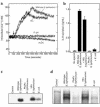
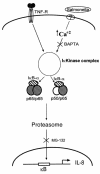
Interactions between the enteric pathogen Salmonella typhimurium and the luminal surface of the intestine provoke an acute inflammatory response, mediated in part by epithelial cell secretion of the chemokine IL-8 and other proinflammatory molecules. This study investigated the mechanism by which this pathogen induces IL-8 secretion in physiologically polarized model intestinal epithelia. IL-8 secretion induced by both the prototypical proinflammatory cytokine TNF-alpha and S. typhimurium was NF-kappaB dependent. However, NF-kappaB activation and IL-8 secretion induced by S. typhimurium, but not by TNF-alpha, was preceded by and required an increase in intracellular [Ca(2+)]. Additionally, agonists that increased intracellular [Ca(2+)] by receptor-dependent (carbachol) or independent (thapsigargin, ionomycin) means also induced IL-8 secretion. Furthermore, the ability of S. typhimurium mutants to induce IkappaB-alpha degradation, NF-kappaB translocation, and IL-8 transcription and secretion correlated precisely with their ability to induce an intracellular [Ca(2+)] increase in model intestinal epithelia, but not with their ability to invade these cells. Finally, S. typhimurium, but not TNF-alpha, induced a Ca(2+)-dependent phosphorylation of IkappaB-alpha. These results indicate that S. typhimurium-induced activation of NF-kappaB-dependent epithelial inflammatory responses proceeds by a Ca(2+)-mediated activation of an IkappaB-alpha kinase. These observations raise the possibility that pharmacologic intervention of the acute inflammatory response can be selectively matched to the specific class of initiating event.
Figures
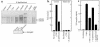
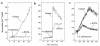


Comment in
-
Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens.J Clin Invest. 2001 Jan;107(1):27-30. doi: 10.1172/JCI11792. J Clin Invest. 2001. PMID: 11134175 Free PMC article. Review. No abstract available.
References
-
- Yamada, T., Alpers, D.H., Owyang, C., Powell, D.W., and Silverstein, F.E. 1995. Textbook of gastroenterology. J.B. Lippincott. Philadelphia, PA. 1606–1629.
-
- McCormick B, Gewirtz A, Madara JL. Epithelial crosstalk with bacteria and immune cells. Curr Opin Gastro. 1998;14:492–497.
-
- McCormick BA, Miller SI, Madara JL. New insights on molecular pathways utilized by salmonella species in cell binding. Front Biosci. 1996;1:d131–d145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

