Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1
- PMID: 10619866
- PMCID: PMC382585
- DOI: 10.1172/JCI7954
Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1
Abstract
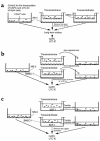
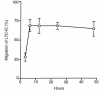
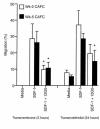
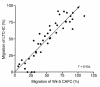
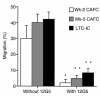
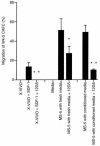
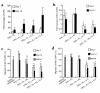
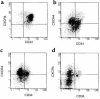
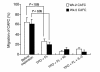
Stromal cell-derived factor-1 (SDF-1) provides a potent chemotactic stimulus for CD34(+) hematopoietic cells. We cultured mobilized peripheral blood (PB) and umbilical cord blood (CB) for up to 5 weeks and examined the migratory activity of cobblestone area-forming cells (CAFCs) and long-term culture-initiating cells (LTC-ICs) in a transwell assay. In this system, SDF-1 or MS-5 marrow stromal cells placed in the lower chamber induced transmembrane and transendothelial migration by 2- and 5-week-old CAFCs and LTC-ICs in 3 hours. Transmigration was blocked by preincubation of input CD34(+) cells with antibody to CXCR4. Transendothelial migration of CB CAFCs and LTC-ICs was higher than that of PB. We expanded CD34(+) cells from CB in serum-free medium with thrombopoietin, flk-2 ligand, and c-kit ligand, with or without IL-3 and found that CAFCs cultured in the absence of IL-3 had a chemotactic response equivalent to noncultured cells, even after 5 weeks. However, addition of IL-3 to the culture reduced this response by 20-50%. These data indicate that SDF-1 induces chemotaxis of primitive hematopoietic cells signaling through CXCR4 and that the chemoattraction could be downmodulated by culture ex vivo.
Figures
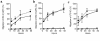
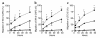
References
-
- Hendrikx PJ, Martens ACM, Hagenbeek A, Keij JF, Visser JWM. Homing of fluorescently labeled murine hematopoietic stem cells. Exp Hematol. 1996;24:129–140. - PubMed
-
- van der Loo JCM, Ploemacher RE. Marrow- and spleen-seeding efficiencies of all murine hematopoietic stem cell subsets are decreased by preincubation with hematopoietic growth factors. Blood. 1995;85:2598–2606. - PubMed
-
- Papayannopoulou T, Craddock C. Homing and trafficking of hemopoietic progenitor cells. Acta Haematol. 1997;97:97–104. - PubMed
-
- Vermeulen M, et al. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

