Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment
- PMID: 10620602
- PMCID: PMC2195801
- DOI: 10.1084/jem.191.1.23
Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment
Abstract
The capacity of precursor B (pre-B) I cells from fetal liver and bone marrow to proliferate and differentiate into surface immunoglobulin-positive immature B cells in vitro was analyzed. Both fetal liver- and bone marrow-derived progenitors do so in a pre-B cell receptor (pre-BCR)-dependent manner in tissue culture medium alone, without addition of other cells or cytokines. Approximately 20% of the initial pre-B I cells enter more than one division. Analyses at the single-cell level show that approximately 15% divide two to five times. Coculture of pre-B I cells with stromal cells did not enhance proliferation or differentiation, whereas the presence of interleukin 7, especially in combination with stromal cells, resulted mainly in the expansion of pre-B I cells and prevented their further differentiation. Thus, the environment of fetal liver or bone marrow is not required for the pre-BCR to exert its function, which is to select and expand cells that have undergone an inframe V(H)-D(H)J(H) rearrangement that produces a pre-BCR-compatible muH chain. It appears unlikely that a ligand for the pre-BCR drives this pre-B cell proliferation.
Figures
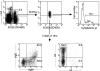

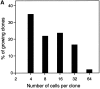


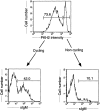
Comment in
-
The development of B cells in the bone marrow is controlled by the balance between cell-autonomous mechanisms and signals from the microenvironment.J Exp Med. 2000 Jan 3;191(1):5-8. doi: 10.1084/jem.191.1.5. J Exp Med. 2000. PMID: 10620600 Free PMC article. No abstract available.
References
-
- Kincade P.W. Formation of B lymphocytes in fetal and adult life. Adv. Immunol. 1981;31:177–245 . - PubMed
-
- Ehlich A., Martin V., Müller W., Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr. Biol. 1994;4:573–583 . - PubMed
-
- ten Boekel E., Melchers F., Rolink A. The status of Ig loci rearrangements in single cells from different stages of B cell development. Int. Immunol. 1995;7:1013–1019 . - PubMed
-
- Melchers F., Rolink A., Grawunder U., Winkler T.H., Karasuyama H., Ghia P., Andersson J. Positively and negatively selecting events during B lymphopoiesis. Curr. Opin. Immunol. 1995;7:214–227 . - PubMed
-
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of λ5 in B cell development. Cell. 1992;69:823–831 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

