Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages
- PMID: 10620610
- PMCID: PMC2195807
- DOI: 10.1084/jem.191.1.115
Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages
Abstract
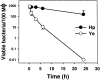
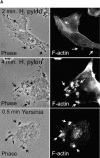
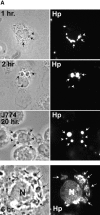
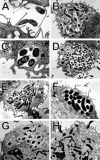
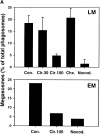
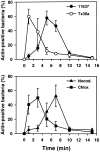
Helicobacter pylori colonizes the gastric epithelium of approximately 50% of the world's population and plays a causative role in the development of gastric and duodenal ulcers. H. pylori is phagocytosed by mononuclear phagocytes, but the internalized bacteria are not killed and the reasons for this host defense defect are unclear. We now show using immunofluorescence and electron microscopy that H. pylori employs an unusual mechanism to avoid phagocytic killing: delayed entry followed by homotypic phagosome fusion. Unopsonized type I H. pylori bound readily to macrophages and were internalized into actin-rich phagosomes after a lag of approximately 4 min. Although early (10 min) phagosomes contained single bacilli, H. pylori phagosomes coalesced over the next approximately 2 h. The resulting "megasomes" contained multiple viable organisms and were stable for 24 h. Phagosome-phagosome fusion required bacterial protein synthesis and intact host microtubules, and both chloramphenicol and nocodazole increased killing of intracellular H. pylori. Type II strains of H. pylori are less virulent and lack the cag pathogenicity island. In contrast to type I strains, type II H. pylori were rapidly ingested and killed by macrophages and did not stimulate megasome formation. Collectively, our data suggest that megasome formation is an important feature of H. pylori pathogenesis.
Figures







References
-
- Czinn S.J., Nedrud J.G. Immunopathology of Helicobacter pylori infection and disease. Springer Semin. Immunopathol. 1997;18:495–513 . - PubMed
-
- Blaser M.J. Helicobacter pylorimicrobiology of a ‘slow’ bacterial infection. Trends Microbiol. 1993;1:255–260 . - PubMed
-
- Telford J.L., Covacci A., Rappuoli R., Ghiara P. Immunobiology of Helicobacter pylori infection. Curr. Opin. Immunol. 1997;9:498–503 . - PubMed
-
- Xiang Z., Censini S., Bayeli P.F., Telford J.L., Figura N., Rappuoli R., Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect. Immun. 1995;63:94–98 . - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

