Pseudorabies virus expressing bovine herpesvirus 1 glycoprotein B exhibits altered neurotropism and increased neurovirulence
- PMID: 10623744
- PMCID: PMC111602
- DOI: 10.1128/jvi.74.2.817-827.2000
Pseudorabies virus expressing bovine herpesvirus 1 glycoprotein B exhibits altered neurotropism and increased neurovirulence
Abstract
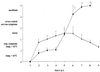
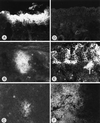
Herpesvirus glycoproteins play dominant roles in the initiation of infection of target cells in culture and thus may also influence viral tropism in vivo. Whereas the relative contribution of several nonessential glycoproteins to neurovirulence and neurotropism of Pseudorabies virus (PrV), an alphaherpesvirus which causes Aujeszky's disease in pigs, has recently been uncovered in studies using viral deletion mutants, the importance of essential glycoproteins is more difficult to assess. We isolated an infectious PrV mutant, PrV-9112C2, which lacks the gene encoding the essential PrV glycoprotein B (gB) but stably carries in its genome and expresses the homologous gene of bovine herpesvirus 1 (BHV-1) (A. Kopp and T. C. Mettenleiter, J. Virol. 66:2754-2762, 1992). Apart from exhibiting a slight delay in penetration kinetics, PrV-9112C2 was similar in its growth characteristics in cell culture to wild-type PrV. To analyze the effect of the exchange of these homologous glycoproteins in PrV's natural host, swine, 4-week-old piglets were intranasally infected with 10(6) PFU of either wild-type PrV strain Kaplan (PrV-Ka), PrV-9112C2, or PrV-9112C2R, in which the PrV gB gene was reinserted instead of the BHV-1 gB gene. Animals infected with PrV-Ka and PrV-9112C2R showed a similar course of disease, i.e., high fever, marked respiratory symptoms but minimal neurological disorders, and excretion of high amounts of virus. All animals survived the infection. In contrast, animals infected with PrV-9112C2 showed no respiratory symptoms and developed only mild fever. However, on day 5 after infection, all piglets developed severe central nervous system (CNS) symptoms leading to death within 48 to 72 h. Detailed histological analyses showed that PrV-9112C2R infected all regions of the nasal mucosa and subsequently spread to the CNS preferentially by the trigeminal route. In contrast, PrV-9112C2 primarily infected the olfactory epithelium and spread via the olfactory route. In the CNS, more viral antigen and significantly more pronounced histological changes resulting in more severe encephalitis were found after PrV-9112C2 infection. Thus, our results demonstrate that replacement of PrV gB by the homologous BHV-1 glycoprotein resulted in a dramatic increase in neurovirulence combined with an alteration in the route of neuroinvasion, indicating that the essential gB is involved in determining neurotropism and neurovirulence of PrV.
Figures


Similar articles
-
Stable rescue of a glycoprotein gII deletion mutant of pseudorabies virus by glycoprotein gI of bovine herpesvirus 1.J Virol. 1992 May;66(5):2754-62. doi: 10.1128/JVI.66.5.2754-2762.1992. J Virol. 1992. PMID: 1313900 Free PMC article.
-
Neuronal pathways of viral invasion in mice after intranasal inoculation of pseudorabies virus PrV-9112C2 expressing bovine herpesvirus 1 glycoprotein B.J Neurovirol. 2006 Feb;12(1):60-4. doi: 10.1080/13550280500516450. J Neurovirol. 2006. PMID: 16595375
-
Glycoprotein gp50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine.J Virol. 1993 Mar;67(3):1529-37. doi: 10.1128/JVI.67.3.1529-1537.1993. J Virol. 1993. PMID: 8382308 Free PMC article.
-
Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine.Microbiol Mol Biol Rev. 2005 Sep;69(3):462-500. doi: 10.1128/MMBR.69.3.462-500.2005. Microbiol Mol Biol Rev. 2005. PMID: 16148307 Free PMC article. Review.
-
Pseudorabies (Aujeszky's disease) virus: state of the art. August 1993.Acta Vet Hung. 1994;42(2-3):153-77. Acta Vet Hung. 1994. PMID: 7810409 Review.
Cited by
-
Characterization and expression of domains of Alphaherpesvirus bovine 1/5 envelope glycoproteins B in Komagataella phaffi.BMC Vet Res. 2023 Jan 31;19(1):28. doi: 10.1186/s12917-023-03590-8. BMC Vet Res. 2023. PMID: 36721143 Free PMC article.
-
Age-Dependent Differences in Pseudorabies Virus Neuropathogenesis and Associated Cytokine Expression.J Virol. 2017 Jan 3;91(2):e02058-16. doi: 10.1128/JVI.02058-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27852848 Free PMC article.
-
Glycoprotein D-independent infectivity of pseudorabies virus results in an alteration of in vivo host range and correlates with mutations in glycoproteins B and H.J Virol. 2001 Nov;75(21):10054-64. doi: 10.1128/JVI.75.21.10054-10064.2001. J Virol. 2001. PMID: 11581374 Free PMC article.
-
Contribution of endocytic motifs in the cytoplasmic tail of herpes simplex virus type 1 glycoprotein B to virus replication and cell-cell fusion.J Virol. 2007 Dec;81(24):13889-903. doi: 10.1128/JVI.01231-07. Epub 2007 Oct 3. J Virol. 2007. PMID: 17913800 Free PMC article.
-
Preparation and Identification of a Monoclonal Antibody against the Pseudorabies Virus gE Glycoprotein through a Novel Strategy.Vet Sci. 2023 Feb 9;10(2):133. doi: 10.3390/vetsci10020133. Vet Sci. 2023. PMID: 36851437 Free PMC article.
References
-
- Babic N, Mettenleiter T C, Ugolini G, Flamand A, Coulon P. Propagation of pseudorabies virus in the nervous system of the mouse after intranasal inoculation. Virology. 1994;204:616–625. - PubMed
-
- Balasch M, Pujols J, Plana-Duran J, Pumarola M. Study of the persistence of Aujeszky's disease (pseudorabies) virus in peripheral blood mononuclear cells and tissues of experimentally infected pigs. Vet Microbiol. 1998;62:171–183. - PubMed
-
- Card J P, Enquist L W. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

