Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system
- PMID: 10623746
- PMCID: PMC111604
- DOI: 10.1128/jvi.74.2.834-845.2000
Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system
Abstract
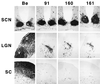
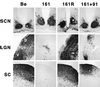
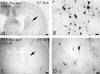
The protein product of the pseudorabies virus (PRV) Us9 gene is a phosphorylated, type II membrane protein that is inserted into virion envelopes and accumulates in the trans-Golgi network. It is among a linked group of three envelope protein genes in the unique short region of the PRV genome which are absent from the attenuated Bartha strain. We found that two different Us9 null mutants exhibited no obvious phenotype after infection of PK15 cells in culture. Unlike those of gE and gI null mutants, the plaque size of Us9 null mutants on Madin-Darby bovine kidney cells was indistinguishable from that of wild-type virus. However, both of the Us9 null mutants exhibited a defect in anterograde spread in the visual and cortical circuitry of the rat. The visual system defect was characterized by restricted infection of a functionally distinct subset of visual projections involved in the temporal organization of behavior, whereas decreased anterograde spread of virus to the cortical projection targets was characteristic of animals receiving direct injections of virus into the cortex. Spread of virus through retrograde pathways in the brain was not compromised by a Us9 deletion. The virulence of the Us9 null mutants, as measured by time to death and appearance of symptoms of infection, also was reduced after their injection into the eye, but not after cortical injection. Through sequence analysis, construction of revertants, measurement of gE and gI protein synthesis in the Us9 null mutants, and mixed-infection studies of rats, we conclude that the restricted-spread phenotype after infection of the rat nervous system reflects the loss of Us9 and is not an indirect effect of the Us9 mutations on expression of glycoproteins gE and gI. Therefore, at least three viral envelope proteins, Us9, gE, and gI, function together to promote efficient anterograde transneuronal infection by PRV in the rat central nervous system.
Figures




References
-
- Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. - PubMed
-
- Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. - PubMed
-
- Ben-Porat T, Kaplan A S. Molecular biology of pseudorabies virus. In: Roizman B, editor. The herpesviruses. New York, N.Y: Plenum Publishing Corp.; 1985. pp. 105–173.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

