Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen
- PMID: 10623795
- PMCID: PMC2239008
- DOI: 10.4049/jimmunol.164.2.562
Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen
Abstract
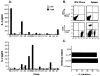
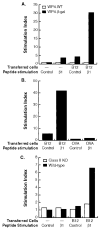
Neoantigens resulting from the inherent genomic instability of tumor cells generally do not trigger immune recognition. Similarly, transfection of tumors with model Ags often fails to elicit CD8+ T cell responses or alter a tumor's growth rate or lethality. We report here that the adoptive transfer of activated Th1-type CD4+ T cells specific for a model tumor Ag results in the de novo generation of CD8+ T cells with specificity to that Ag and concomitant tumor destruction. The anti-tumor effects of the CD4+ T cells required the presence of both MHC class I and class II on host cells, as evidenced by experiments in knockout mice, suggesting that CD4+ T cells enhanced the ability of host APC to activate endogenous CD8+ T cells. These results indicate that the apparent inability of tumor cells expressing highly immunogenic epitopes to activate tumor-specific CD8+ T cells can be altered by activated CD4+ T cells.
Figures

References
-
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

