Use of the triazolotriazine [3H]ZM 241385 as a radioligand at recombinant human A2B adenosine receptors
- PMID: 10624567
- PMCID: PMC3425640
Use of the triazolotriazine [3H]ZM 241385 as a radioligand at recombinant human A2B adenosine receptors
Abstract
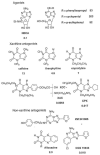
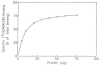
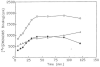
Radiolabeled ZM 241385 (4-(2-[7-amino-2- ¿furyl¿¿1,2,4¿triazolo¿2,3-a¿¿1,3,5¿triazin-5-ylaminoethyl)p henol), has previously been used as a high affinity radioligand for the labeling of A2A adenosine receptors in cell membranes. Another subtype, the A2B receptor, is the least well-defined subtype of adenosine receptors and lacks selective pharmacological probes. In the present study, we have used [3H]ZM 241385 as a radioligand to label recombinant human A2B adenosine receptors in HEK-293 cell membranes, that do not express A2A adenosine receptors, and found that the pharmacological profile is consistent with the SAR of A2B receptors. Saturable, specific binding (Kd 33.6 nM, Bmax 4.48 pmol/mg protein) that was best described by a one-site model was found, and specific binding was approximately 75% of total binding. [3H]ZM 241385 binding was displaceable by a large number of compounds known to interact with A2B receptors; thus, this method has promise as a tool in the search for agonists and antagonists selective for this subtype. Xanthine analogs, which are antagonists, proved to be the most potent displacers. The Ki of XAC, xanthine amine congener, was 12.3 nM, while CPX (8-cyclopentyl-1,3-dipropylxanthine) was less potent. The non-selective triazoloquinazoline antagonist CGS 15943 (Ki 16.4 nM), which is similar in structure to ZM 241385, was slightly less potent than XAC. The non-xanthine A2B-antagonist alloxazine displaced [3H]ZM 241385-binding with a Ki of 462 nM, similar to its affinity in functional assays. Adenosine derivatives known to activate this receptor subtype, such as NECA (5'-N-ethylcarboxamidoadenosine) and R-PIA (N6-phenylisopropyladenosine), were considerably less potent than the 8-substituted xanthines examined.
Figures


References
-
- Linden J, Jacobson KA. Molecular biology of recombinant adenosine receptors. In: Burnstock G, Dobson JG, Liang BT, Linden J, editors. Cardiovascular Bioloy of Purines. Kluwer; 1998. pp. 1–20.
-
- Feoktistov I, Murray JJ, Biaggioni I. Positive modulation of intracellular Ca2+ by adenosine A2b, prostacyclin and prostaglandin E2 reeeptors via a cholera toxin-sensitive mechanism in human erythroleukemia cells. Mol. Pharmalcol. 1994;45:1160–1167. - PubMed
-
- Feoktistov I, Ind Bilggioni I. Adenosine A2B ReceptOrs. Pharmacol Rev. 1997;49:381–402. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
