Inhibitory region of troponin I: Ca(2+)-dependent structural and environmental changes in the troponin-tropomyosin complex and in reconstituted thin filaments
- PMID: 10625482
- PMCID: PMC7001381
- DOI: 10.1021/bi991903b
Inhibitory region of troponin I: Ca(2+)-dependent structural and environmental changes in the troponin-tropomyosin complex and in reconstituted thin filaments
Abstract
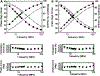
In muscle thin filaments, the inhibitory region (residues 96-117) of troponin I (TnI) is thought to interact with troponin C (TnC) in the presence of Ca(2+) and with actin in the absence of Ca(2+). To better understand these interactions, we prepared mutant TnIs which contained a single Cys-96 or Cys-117 and labeled them with the thiol-specific fluorescent probe N-(iodoacetyl)-N'-(1-sulfo-5-naphthyl)ethylenediamine (IAEDANS). We characterized the microenvironments of the AEDANS labels on TnI in the presence and absence of Ca(2+) by measuring the extent of acrylamide quenching of fluorescence and lifetime-resolved anisotropy. In the troponin-tropomyosin (Tn-Tm) complex, the AEDANS labels on both Cys-96 and Cys-117 were less accessible to solvent and less flexible in the presence of Ca(2+), reflecting closer interactions with TnC under these conditions. In reconstituted thin filaments, the environment of the AEDANS on Cys-96 was not greatly affected by Ca(2+), while the AEDANS on Cys-117 was more accessible but significantly less flexible as it moved away from actin and interacted strongly with TnC in the presence of Ca(2+). We used fluorescence resonance energy transfer (FRET) to measure distances between AEDANS on TnI Cys-96 or Cys-117 and 4-¿[(dimethylamino)phenyl]azo¿phenyl-4'-maleimide (DABmal) on actin Cys-374 in reconstituted thin filaments. In the absence of Ca(2+), the mean distances were 40.2 A for Cys-96 and 35.2 A for Cys-117. In the presence of Ca(2+), Cys-96 moved away from actin Cys-374 by approximately 3.6 A, while Cys-117 moved away by approximately 8 A. This suggests the existence of a flexible "hinge" region near the middle of TnI, allowing amino acid residues in the N-terminal half of TnI to interact with TnC in a Ca(2+)-independent manner, while the C-terminal half of TnI binds to actin in the absence of Ca(2+) or to TnC in the presence of Ca(2+). This is the first report to demonstrate structural movement of the inhibitory region of TnI in the thin filament.
Figures

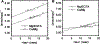
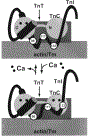
References
-
- Leavis PC, and Gergely J. (1984) CRC Crit. Rev. Biochem 16, 235–305. - PubMed
-
- Zot A, and Potter JD (1987) Annu. Rev. Biophys. Biophys. Chem 16, 535–559. - PubMed
-
- Farah C, and Reinach FC (1995) FASEB J. 9, 755–767. - PubMed
-
- Tobacman LS (1996) Annu. Rev. Physiol 58, 447–481. - PubMed
-
- Squire JM, and Morris EP (1998) FASEB J. 12, 761–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

