Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases
- PMID: 10627532
- PMCID: PMC111456
- DOI: 10.1128/jvi.74.3.1224-1233.2000
Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases
Abstract
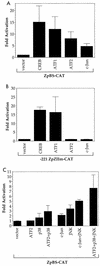
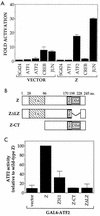
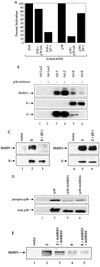
Expression of either Epstein-Barr virus (EBV) immediate-early protein BZLF1 (Z) or BRLF1 (R) is sufficient to convert EBV infection from the latent to lytic form. Disruption of viral latency requires transcriptional activation of the Z and R promoters. The Z and R proteins are transcriptional activators, and each immediate-early protein activates expression of the other immediate-early protein. Z activates the R promoter through a direct binding mechanism. However, R does not bind directly to the Z promoter. In this study, we demonstrate that the ZII element (a cyclic AMP response element site) in the Z promoter is required for efficient activation by R. The ZII element has been shown to be important for induction of lytic EBV infection by tetradecanoyl phorbol acetate and surface immunoglobulin cross-linking and is activated by Z through an indirect mechanism. We demonstrate that both R and Z activate the cellular stress mitogen-activated protein (MAP) kinases, p38 and JNK, resulting in phosphorylation (and activation) of the cellular transcription factor ATF2. Furthermore, we show that the ability of R to induce lytic EBV infection in latently infected cells is significantly reduced by inhibition of either the p38 kinase or JNK pathways. In contrast, inhibition of stress MAP kinase pathways does not impair the ability of Z expression vectors to disrupt viral latency, presumably because expression of Z under the control of a strong heterologous promoter bypasses the need to activate Z transcription. Thus, both R and Z can activate the Z promoter indirectly by inducing ATF2 phosphorylation, and this activity appears to be important for R-induced disruption of viral latency.
Figures





References
-
- Adamson A L, Kenney S C. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology. 1998;251:187–197. - PubMed
-
- Cen H, McKnight J L C. EBV-immortalized isogenic human B-cell clones exhibit differences in DNA-protein complex formation on the BZLF1 and BRLF1 promoter regions among latent, lytic and TPA-activated cell lines. Virus Res. 1994;31:89–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

