Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway
- PMID: 10627563
- PMCID: PMC111487
- DOI: 10.1128/jvi.74.3.1513-1523.2000
Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway
Abstract
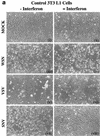
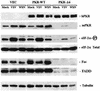
Interferon (IFN) mediates its antiviral effects by inducing a number of responsive genes, including the double-stranded RNA (dsRNA)-dependent protein kinase, PKR. Here we report that inducible overexpression of functional PKR in murine fibroblasts sensitized cells to apoptosis induced by influenza virus, while in contrast, cells expressing a dominant-negative variant of PKR were completely resistant. We determined that the mechanism of influenza virus-induced apoptosis involved death signaling through FADD/caspase-8 activation, while other viruses such as vesicular stomatitis virus (VSV) and Sindbis virus (SNV) did not significantly provoke PKR-mediated apoptosis but did induce cytolysis of fibroblasts via activation of caspase-9. Significantly, treatment with IFN-alpha/beta greatly sensitized the fibroblasts to FADD-dependent apoptosis in response to dsRNA treatment or influenza virus infection but completely protected the cells against VSV and SNV replication in the absence of any cellular destruction. The mechanism by which IFN increases the cells' susceptibility to lysis by dsRNA or certain virus infection is by priming cells to FADD-dependent apoptosis, possibly by regulating the activity of the death-induced signaling complex (DISC). Conversely, IFN is also able to prevent the replication of viruses such as VSV that avoid triggering FADD-mediated DISC activity, by noncytopathic mechanisms, thus preventing destruction of the cell.
Figures


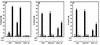
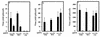
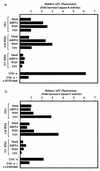
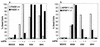
References
-
- Abraham N, Stojl D F, Duncan P I, Methot N, Ishii T, Dube M, Vanderhyden B C, Atkins H L, Gray D A, McBurney M W, Koromilas A E, Brown E G, Sonenberg N, Bell J C. Characterization of transgenic mice with a targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;247:5953–5962. - PubMed
-
- Chinnaiyan A M, O'Rouke K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

