Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks
- PMID: 10627582
- PMCID: PMC6774099
- DOI: 10.1523/JNEUROSCI.20-01-00066.2000
Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks
Abstract
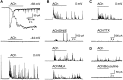
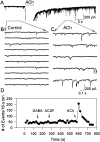
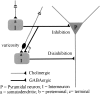
Cholinergic control of the activity of human cerebral cortical circuits has long been thought to be accounted for by the interaction of acetylcholine (ACh) with muscarinic receptors. Here we report the discovery of functional nicotinic receptors (nAChRs) in interneurons of the human cerebral cortex and discuss the physiological and clinical implications of these findings. The whole-cell mode of the patch-clamp technique was used to record responses triggered by U-tube application of the nonselective agonist ACh and of the alpha7-nAChR-selective agonist choline to interneurons visualized by means of infrared-assisted videomicroscopy in slices of the human cerebral cortex. Choline induced rapidly desensitizing whole-cell currents that, being sensitive to blockade by methyllycaconitine (MLA; 50 nM), were most likely subserved by an alpha7-like nAChR. In contrast, ACh evoked slowly decaying whole-cell currents that, being sensitive to blockade by dihydro-beta-erythroidine (DHbetaE; 10 microM), were most likely subserved by an alpha4beta2-like nAChR. Application of ACh (but not choline) to the slices also triggered GABAergic postsynaptic currents (PSCs). Evidence is provided that ACh-evoked PSCs are the result of activation of alpha4beta2-like nAChRs present in preterminal axon segments and/or in presynaptic terminals of interneurons. Thus, nAChRs can relay inhibitory and/or disinhibitory signals to pyramidal neurons and thereby modulate the activity of neuronal circuits in the human cerebral cortex. These mechanisms, which appear to be retained across species, can account for the involvement of nAChRs in cognitive functions and in certain neuropathological conditions.
Figures





References
-
- Albuquerque EX, Alkondon M, Pereira EFR, Castro NG, Schrattenholz A, Barbosa CTF, Bonfante-Cabarcas R, Aracava Y, Eisenberg H, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1996;280:1117–1136. - PubMed
-
- Alkondon M, Pereira EFR, Barbosa CTF, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates γ-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. - PubMed
-
- Alkondon M, Pereira EFR, Albuquerque EX. α-Bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
