Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase
- PMID: 10627585
- PMCID: PMC6774102
- DOI: 10.1523/JNEUROSCI.20-01-00089.2000
Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase
Abstract
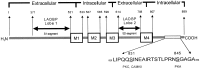
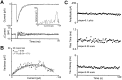
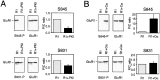
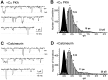
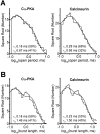
Modulation of postsynaptic AMPA receptors in the brain by phosphorylation may play a role in the expression of synaptic plasticity at central excitatory synapses. It is known from biochemical studies that GluR1 AMPA receptor subunits can be phosphorylated within their C terminal by cAMP-dependent protein kinase A (PKA), which is colocalized with the phosphatase calcineurin (i.e., phosphatase 2B). We have examined the effect of PKA and calcineurin on the time course, peak open probability (P(O, PEAK)), and single-channel properties of glutamateevoked responses for neuronal AMPA receptors and homomeric GluR1(flip) receptors recorded in outside-out patches. Inclusion of purified catalytic subunit Calpha-PKA in the pipette solution increased neuronal AMPA receptor P(O,PEAK) (0.92) compared with recordings made with calcineurin included in the pipette (P(O,PEAK) 0.39). Similarly, Calpha-PKA increased P(O,PEAK) for recombinant GluR1 receptors (0. 78) compared with patches excised from cells cotransfected with a cDNA encoding the PKA peptide inhibitor PKI (P(O,PEAK) 0.50) or patches with calcineurin included in the pipette (P(O,PEAK) 0.42). Neither PKA nor calcineurin altered the amplitude of single-channel subconductance levels, weighted mean unitary current, mean channel open period, burst length, or macroscopic response waveform for recombinant GluR1 receptors. Substitution of an amino acid at the PKA phosphorylation site (S845A) on GluR1 eliminated the PKA-induced increase in P(O,PEAK), whereas the mutation of a Ca(2+), calmodulin-dependent kinase II and PKC phosphorylation site (S831A) was without effect. These results suggest that AMPA receptor peak response open probability can be increased by PKA through phosphorylation of GluR1 Ser845.
Figures




References
-
- Asztely F, Gustafsson B. Ionotropic glutamate receptors. Their possible role in the expression of hippocampal synaptic plasticity. Mol Neurobiol. 1996;12:1–11. - PubMed
-
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. - PubMed
-
- Benke TA, Luthi A, Isaac JTR, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
