Ninjurin2, a novel homophilic adhesion molecule, is expressed in mature sensory and enteric neurons and promotes neurite outgrowth
- PMID: 10627596
- PMCID: PMC6774113
- DOI: 10.1523/JNEUROSCI.20-01-00187.2000
Ninjurin2, a novel homophilic adhesion molecule, is expressed in mature sensory and enteric neurons and promotes neurite outgrowth
Abstract
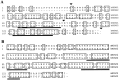
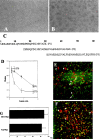
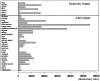
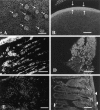
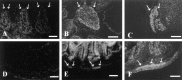
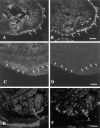
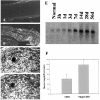
A large number of cell adhesion molecules mediate cell-to-cell and cell-to-extracellular matrix interaction during development, differentiation and regeneration of the peripheral nervous system. Here, we report the identification of a novel cell surface adhesion molecule, ninjurin2 (for nerve injury induced protein 2). Ninjurin2 is a homolog of a homophilic cellular adhesion molecule, ninjurin1, that was previously isolated as a gene induced in Schwann cells after nerve injury. Ninjurin1 and 2 share conserved hydrophobic regions for their transmembrane domains; however, they do not contain comparable adhesion motifs nor do they interact with each other. In the peripheral nervous system, ninjurin2 is expressed constitutively in mature sensory and enteric neurons but not in glial cells or in autonomic ganglia. Ninjurin2 is upregulated in Schwann cells surrounding the distal segment of injured nerve with a time course similar to that of ninjurin1, neural CAM, and L1. Ninjurin2 promotes neurite outgrowth from primary cultured dorsal root ganglion neurons, presumably via homophilic cellular interactions. Ninjurin2 is also highly expressed in hematopoietic and lymphatic tissues. Finally, the ninjurin2 gene is located on human chromosome 12p13 in which several disorders of unknown etiology have been mapped, including inflammatory bowel disease and acrocallosal syndrome.
Figures


References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17:353–361. - PubMed
-
- Araki T, Zimonjic DB, Popescu NC, Milbrandt J. Mechanism of homophilic binding mediated by ninjurin, a novel widely expressed adhesion molecule. J Biol Chem. 1997;272:21373–21380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
