The bHLH gene hes1 as a repressor of the neuronal commitment of CNS stem cells
- PMID: 10627606
- PMCID: PMC6774123
- DOI: 10.1523/JNEUROSCI.20-01-00283.2000
The bHLH gene hes1 as a repressor of the neuronal commitment of CNS stem cells
Abstract
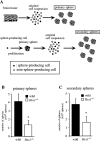
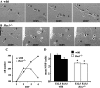
Hes1 is one of the basic helix-loop-helix transcription factors that regulate mammalian CNS development, and its loss- and gain-of-function phenotypes indicate that it negatively regulates neuronal differentiation. Here we report that Hes1(-/-) mice expressed both early (TuJ1 and Hu) and late (MAP2 and Neurofilament) neuronal markers prematurely, and that there were approximately twice the normal number of neurons in the Hes1(-/-) brain during early neural development. However, immunochemical analyses of sections and dissociated cells using neural progenitor markers, including nestin, failed to detect any changes in Hes1(-/-) progenitor population. Therefore, further characterization of neural progenitor cells that discriminated between multipotent and monopotent cells was performed using two culture methods, low-density culture, and a neurosphere assay. We demonstrate that the self-renewal activity of multipotent progenitor cells was reduced in the Hes1(-/-) brain, and that their subsequent commitment to the neuronal lineage was accelerated. The Hes1(-/-) neuronal progenitor cells were functionally abnormal, in that they divided, on average, only once, and then generated two neurons, (instead of one progenitor cell and one neuron), whereas wild-type progenitor cells divided more. In addition, some Hes1(-/-) progenitors followed an apoptotic fate. The overproduction of neurons in the early Hes1(-/-) brains may reflect this premature and immediate generation of neurons as well as a net increase in the number of neuronal progenitor cells. Taken together, we conclude that Hes1 is important for maintaining the self-renewing ability of progenitors and for repressing the commitment of multipotent progenitor cells to a neuronal fate, which is critical for the correct number of neurons to be produced and for the establishment of normal neuronal function.
Figures


References
-
- Akazawa C, Sasai Y, Nakanishi S, Kageyama R. Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J Biol Chem. 1992;267:21879–21885. - PubMed
-
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Bayer SA, Altman J. Neocortical development. Raven; New York: 1991.
-
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
