Membrane potential and firing rate in cat primary visual cortex
- PMID: 10627623
- PMCID: PMC6774139
- DOI: 10.1523/JNEUROSCI.20-01-00470.2000
Membrane potential and firing rate in cat primary visual cortex
Abstract
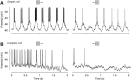
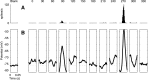
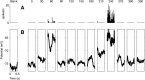
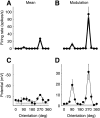
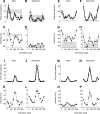
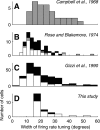
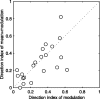
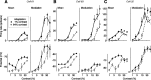
We have investigated the relationship between membrane potential and firing rate in cat visual cortex and found that the spike threshold contributes substantially to the sharpness of orientation tuning. The half-width at half-height of the tuning of the spike responses was 23 +/- 8 degrees, compared with 38 +/- 15 degrees for the membrane potential responses. Direction selectivity was also greater in spike responses (direction index, 0.61 +/- 0.35) than in membrane potential responses (0.28 +/- 0.21). Threshold also increased the distinction between simple and complex cells, which is commonly based on the linearity of the spike responses to drifting sinusoidal gratings. In many simple cells, such stimuli evoked substantial elevations in the mean potential, which are nonlinear. Being subthreshold, these elevations would be hard to detect in the firing rate responses. Moreover, just as simple cells displayed various degrees of nonlinearity, complex cells displayed various degrees of linearity. We fitted the firing rates with a classic rectification model in which firing rate is zero at potentials below a threshold and grows linearly with the potential above threshold. When the model was applied to a low-pass-filtered version of the membrane potential (with spikes removed), the estimated values of threshold (-54.4 +/- 1.4 mV) and linear gain (7.2 +/- 0.6 spikes. sec(-1). mV(-1)) were similar across the population. The predicted firing rates matched the observed firing rates well and accounted for the sharpening of orientation tuning of the spike responses relative to that of the membrane potential. As it was for stimulus orientation, threshold was also independent of stimulus contrast. The rectification model accounted for the dependence of spike responses on contrast and, because of a stimulus-induced tonic hyperpolarization, for the response adaptation induced by prolonged stimulation. Because gain and threshold are unaffected by visual stimulation and by adaptation, we suggest that they are constant under all conditions.
Figures





References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. - PubMed
-
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Whitteridge D. Estimates of the net excitatory currents evoked by visual stimulation of identified neurons in cat visual cortex. Cereb Cortex. 1998;8:462–476. - PubMed
-
- Albrecht DG, Geisler WS. Motion sensitivity and the contrast-response function of simple cells in the visual cortex. Vis Neurosci. 1991;7:531–546. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
