Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice
- PMID: 10629031
- PMCID: PMC85191
- DOI: 10.1128/MCB.20.3.755-759.2000
Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice
Abstract
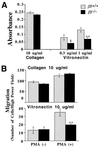
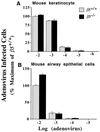
Integrins have been shown to play important roles in embryonic development, wound healing, metastasis, and other biological processes. alphavbeta5 is a receptor for RGD-containing extracellular matrix proteins that has been suggested to be important in cutaneous wound healing and adenovirus infection. To examine the in vivo function of this receptor, we have generated mice lacking beta5 expression, using homologous recombination in embryonic stem cells. Mice homozygous for a null mutation of the beta5 subunit gene develop, grow, and reproduce normally. Keratinocytes harvested from beta5(-/-) mice demonstrate impaired migration on and adhesion to the alphavbeta5 ligand, vitronectin. However, the rate of healing of cutaneous wounds is not different in beta5(-/-) and beta5(+/+) mice. Furthermore, keratinocytes and airway epithelial cells obtained from null mice show adenovirus infection efficiency equal to that from wild-type mice. These data suggest that alphavbeta5 is not essential for normal development, reproduction, adenovirus infection, or the healing of cutaneous wounds.
Figures

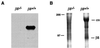
References
-
- Bader B L, Rayburn H, Crowley D, Hynes R O. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. - PubMed
-
- Breuss J M, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521–1527. - PubMed
-
- Busk M, Pytela R, Sheppard D. Characterization of the integrin αvβ6 as a fibronectin-binding protein. J Biol Chem. 1992;267:5790–5796. - PubMed
-
- Capecchi M R. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
