The function of DNA polymerase alpha at telomeric G tails is important for telomere homeostasis
- PMID: 10629035
- PMCID: PMC85195
- DOI: 10.1128/MCB.20.3.786-796.2000
The function of DNA polymerase alpha at telomeric G tails is important for telomere homeostasis
Abstract
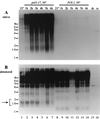
Telomere length control is influenced by several factors, including telomerase, the components of telomeric chromatin structure, and the conventional replication machinery. Although known components of the replication machinery can influence telomere length equilibrium, little is known about why mutations in certain replication proteins cause dramatic telomere lengthening. To investigate the cause of telomere elongation in cdc17/pol1 (DNA polymerase alpha) mutants, we examined telomeric chromatin, as measured by its ability to repress transcription on telomere-proximal genes, and telomeric DNA end structures in pol1-17 mutants. pol1-17 mutants with elongated telomeres show a dramatic loss of the repression of telomere-proximal genes, or telomeric silencing. In addition, cdc17/pol1 mutants grown under telomere-elongating conditions exhibit significant increases in single-stranded character in telomeric DNA but not at internal sequences. The single strandedness is manifested as a terminal extension of the G-rich strand (G tails) that can occur independently of telomerase, suggesting that cdc17/pol1 mutants exhibit defects in telomeric lagging-strand synthesis. Interestingly, the loss of telomeric silencing and the increase in the sizes of the G tails at the telomeres temporally coincide and occur before any detectable telomere lengthening is observed. Moreover, the G tails observed in cdc17/pol1 mutants incubated at the semipermissive temperature appear only when the cells pass through S phase and are processed by the time cells reach G(1). These results suggest that lagging-strand synthesis is coordinated with telomerase-mediated telomere maintenance to ensure proper telomere length control.
Figures

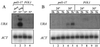





References
-
- Aparicio O M, Billington B L, Gottschling D E. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;66:1279–1287. - PubMed
-
- Aparicio O M, Gottschling D E. Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 1994;8:1133–1146. - PubMed
-
- Astell C R, Ahlstrom-Jonasson L, Smith M. The sequence of the DNAs coding for the mating-type loci of Saccharomyces cerevisiae. Cell. 1981;27:15–23. - PubMed
-
- Beeler T, Gable K, Zhao C, Dunn T. A novel protein, CSG2p, is required for Ca2+ regulation in Saccharomyces cerevisiae. J Biol Chem. 1994;269:7279–7284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
