Biochemical and genetic conservation of fission yeast Dsk1 and human SR protein-specific kinase 1
- PMID: 10629038
- PMCID: PMC85198
- DOI: 10.1128/MCB.20.3.816-824.2000
Biochemical and genetic conservation of fission yeast Dsk1 and human SR protein-specific kinase 1
Abstract
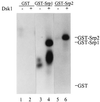
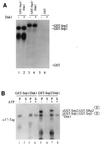
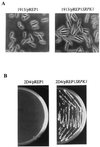
Arginine/serine-rich (RS) domain-containing proteins and their phosphorylation by specific protein kinases constitute control circuits to regulate pre-mRNA splicing and coordinate splicing with transcription in mammalian cells. We present here the finding that similar SR networks exist in Schizosaccharomyces pombe. We previously showed that Dsk1 protein, originally described as a mitotic regulator, displays high activity in phosphorylating S. pombe Prp2 protein (spU2AF59), a homologue of human U2AF65. We now demonstrate that Dsk1 also phosphorylates two recently identified fission yeast proteins with RS repeats, Srp1 and Srp2, in vitro. The phosphorylated proteins bear the same phosphoepitope found in mammalian SR proteins. Consistent with its substrate specificity, Dsk1 forms kinase-competent complexes with those proteins. Furthermore, dsk1(+) gene determines the phenotype of prp2(+) overexpression, providing in vivo evidence that Prp2 is a target for Dsk1. The dsk1-null mutant strain became severely sick with the additional deletion of a related kinase gene. Significantly, human SR protein-specific kinase 1 (SRPK1) complements the growth defect of the double-deletion mutant. In conjunction with the resemblance of dsk1(+) and SRPK1 in sequence homology, biochemical properties, and overexpression phenotypes, the complementation result indicates that SRPK1 is a functional homologue of Dsk1. Collectively, our studies illustrate the conserved SR networks in S. pombe consisting of RS domain-containing proteins and SR protein-specific kinases and thus establish the importance of the networks in eucaryotic organisms.
Figures




Similar articles
-
Interactions between two fission yeast serine/arginine-rich proteins and their modulation by phosphorylation.Biochem J. 2002 Dec 1;368(Pt 2):527-34. doi: 10.1042/BJ20021133. Biochem J. 2002. PMID: 12186627 Free PMC article.
-
Fission yeast mitotic regulator Dsk1 is an SR protein-specific kinase.J Biol Chem. 1998 Mar 6;273(10):5963-9. doi: 10.1074/jbc.273.10.5963. J Biol Chem. 1998. PMID: 9488736
-
Phosphoproteomics Reveals Novel Targets and Phosphoprotein Networks in Cell Cycle Mediated by Dsk1 Kinase.J Proteome Res. 2020 Apr 3;19(4):1776-1787. doi: 10.1021/acs.jproteome.0c00027. Epub 2020 Mar 5. J Proteome Res. 2020. PMID: 32062975
-
Phosphorylation mechanism and structure of serine-arginine protein kinases.FEBS J. 2011 Feb;278(4):587-97. doi: 10.1111/j.1742-4658.2010.07992.x. Epub 2011 Jan 12. FEBS J. 2011. PMID: 21205204 Free PMC article. Review.
-
Multiple genetic and biochemical interactions of Brr2, Prp8, Prp31, Prp1 and Prp4 kinase suggest a function in the control of the activation of spliceosomes in Schizosaccharomyces pombe.Curr Genet. 2005 Sep;48(3):151-61. doi: 10.1007/s00294-005-0013-6. Epub 2005 Oct 12. Curr Genet. 2005. PMID: 16133344 Review.
Cited by
-
The Dual-Specificity LAMMER Kinase Affects Stress-Response and Morphological Plasticity in Fungi.Front Cell Infect Microbiol. 2019 Jun 19;9:213. doi: 10.3389/fcimb.2019.00213. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31275866 Free PMC article. Review.
-
Regulated cellular partitioning of SR protein-specific kinases in mammalian cells.Mol Biol Cell. 2006 Feb;17(2):876-85. doi: 10.1091/mbc.e05-10-0963. Epub 2005 Nov 30. Mol Biol Cell. 2006. PMID: 16319169 Free PMC article.
-
trans and cis splicing in trypanosomatids: mechanism, factors, and regulation.Eukaryot Cell. 2003 Oct;2(5):830-40. doi: 10.1128/EC.2.5.830-840.2003. Eukaryot Cell. 2003. PMID: 14555465 Free PMC article. Review. No abstract available.
-
Interactions between two fission yeast serine/arginine-rich proteins and their modulation by phosphorylation.Biochem J. 2002 Dec 1;368(Pt 2):527-34. doi: 10.1042/BJ20021133. Biochem J. 2002. PMID: 12186627 Free PMC article.
-
Gene ontology: tool for the unification of biology. The Gene Ontology Consortium.Nat Genet. 2000 May;25(1):25-9. doi: 10.1038/75556. Nat Genet. 2000. PMID: 10802651 Free PMC article.
References
-
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with fission yeast: a laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993.
-
- Colwill K, Feng L L, Yeakley J M, Gish G D, Caceres J F, Pawson T, Fu X D. SRPK1 and Clk/Sty protein kinases show distinct substrate specificities for serine/arginine-rich splicing factors. J Biol Chem. 1996;271:24569–24575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
