The Cbl proto-oncogene product negatively regulates the Src-family tyrosine kinase Fyn by enhancing its degradation
- PMID: 10629042
- PMCID: PMC85202
- DOI: 10.1128/MCB.20.3.851-867.2000
The Cbl proto-oncogene product negatively regulates the Src-family tyrosine kinase Fyn by enhancing its degradation
Abstract
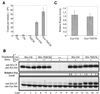
Fyn is a prototype Src-family tyrosine kinase that plays specific roles in neural development, keratinocyte differentiation, and lymphocyte activation, as well as roles redundant with other Src-family kinases. Similar to other Src-family kinases, efficient regulation of Fyn is achieved through intramolecular binding of its SH3 and SH2 domains to conserved regulatory regions. We have investigated the possibility that the tyrosine kinase regulatory protein Cbl provides a complementary mechanism of Fyn regulation. We show that Cbl overexpression in 293T embryonic kidney and Jurkat T-lymphocyte cells led to a dramatic reduction in the active pool of Fyn; this was seen as a reduction in Fyn autophosphorylation, reduced phosphorylation of in vivo substrates, and inhibition of transcription from a Src-family kinase response element linked to a luciferase reporter. Importantly, a Fyn mutant (FynY528F) relieved of intramolecular repression was still negatively regulated by Cbl. The Cbl-dependent negative regulation of Fyn did not appear to be mediated by inhibition of Fyn kinase activity but was correlated with enhanced protein turnover. Consistent with such a mechanism, elevated levels of Fyn protein were observed in cell lines derived from Cbl(-/-) mice compared to those in wild-type controls. The effects of Cbl on Fyn were not observed when the 70ZCbl mutant protein was analyzed. Taken together, these observations implicate Cbl as a component in the negative regulation of Fyn and potentially other Src-family kinases, especially following kinase activation. These results also suggest that protein degradation may be a general mechanism for Cbl-mediated negative regulation of activated tyrosine kinases.
Figures








References
-
- Alexandropoulos K, Baltimore D. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 1996;10:1341–1355. - PubMed
-
- Anderson S M, Burton E A, Koch B L. Phosphorylation of Cbl following stimulation with interleukin-3 and its association with Grb2, Fyn, and phosphatidylinositol 3-kinase. J Biol Chem. 1997;272:739–745. - PubMed
-
- Andoniou C E, Thien C B, Langdon W Y. The two major sites of cbl tyrosine phosphorylation in abl-transformed cells select the crkL SH2 domain. Oncogene. 1996;12:1981–1989. - PubMed
-
- Blake T J, Shapiro M, Morse H C, Langdon W Y. The sequences of the human and mouse c-cbl proto-oncogenes show v-cbl was generated by a large truncation encompassing a proline-rich domain and a leucine zipper-like motif. Oncogene. 1991;6:653–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
