Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane
- PMID: 10629050
- PMCID: PMC85210
- DOI: 10.1128/MCB.20.3.929-935.2000
Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane
Abstract
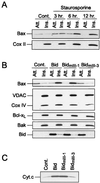
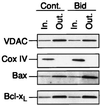
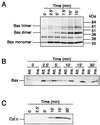
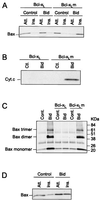
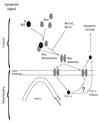
In many types of apoptosis, the proapoptotic protein Bax undergoes a change in conformation at the level of the mitochondria. This event always precedes the release of mitochondrial cytochrome c, which, in the cytosol, activates caspases through binding to Apaf-1. The mechanisms by which Bax triggers cytochrome c release are unknown. Here we show that following binding to the BH3-domain-only proapoptotic protein Bid, Bax oligomerizes and then integrates in the outer mitochondrial membrane, where it triggers cytochrome c release. Bax mitochondrial membrane insertion triggered by Bid may represent a key step in pathways leading to apoptosis.
Figures


References
-
- Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. - PubMed
-
- Antonsson B, Conti F, Ciavatta A M, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J-J, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou J-C. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
