Mutational analysis of a role for salicylic acid in iron metabolism of Mycobacterium smegmatis
- PMID: 10629169
- PMCID: PMC94272
- DOI: 10.1128/JB.182.2.264-271.2000
Mutational analysis of a role for salicylic acid in iron metabolism of Mycobacterium smegmatis
Abstract
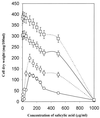
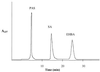
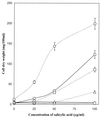
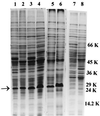
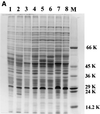
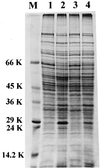
The role of salicylic acid in iron metabolism was examined in two wild-type strains (mc(2)155 and NCIMB 8548) and three mutant strains (mc(2)1292 [lacking exochelin], SM3 [lacking iron-dependent repressor protein IdeR] and S99 [a salicylate-requiring auxotroph derived in this study]) of Mycobacterium smegmatis. Synthesis of salicylate in SM3 was derepressed even in the presence of iron, as was synthesis of the siderophores exochelin, mycobactin, and carboxymycobactin. S99 was dependent on salicylate for growth and failed to grow with the three ferrisiderophores, suggesting that salicylate fulfills an additional function(s) other than being a precursor of mycobactin and carboxymycobactin. Salicylic acid at 100 microgram/ml repressed the formation of a 29-kDa cell envelope protein (putative exochelin receptor protein) in S99 grown both iron deficiently and iron sufficiently. In contrast, synthesis of this protein was affected only under iron-limited conditions in the parent strain, mc(2)155, and remained unaltered in SM3, suggesting an interaction between the IdeR protein and salicylate. Thus, salicylate may also function as a signal molecule for recognition of cellular iron status. Growth of all strains and mutants with p-aminosalicylate (PAS) at 100 microgram/ml increased salicylate accumulation between three- and eightfold under both iron-limited and iron-sufficient growth conditions and decreased mycobactin accumulation by 40 to 80% but increased carboxymycobactin accumulation by 50 to 55%. Thus, although PAS inhibited salicylate conversion to mycobactin, presumptively by blocking salicylate AMP kinase, PAS also interferes with the additional functions of salicylate, as its effect was heightened in S99 when the salicylate concentration was minimal.
Figures


References
-
- Barclay R, Wheeler P R. Metabolism of mycobacteria in tissues. In: Ratledge C, Stanford J L, Grange J M, editors. The biology of the mycobacteria. Vol. 3. London, United Kingdom: Academic Press Ltd.; 1989. pp. 137–196.
-
- Brown K A, Ratledge C. The effect of p-aminosalicylic acid on iron transport and assimilation in mycobacteria. Biochim Biophys Acta. 1975;385:207–220. - PubMed
-
- Calder K M, Horwitz M A. Identification of iron-regulated proteins of Mycobacterium tuberculosis and cloning of tandem genes encoding a low iron-induced protein and a metal transporting ATPase with similarities to two-component metal transport systems. Microb Pathog. 1998;24:133–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

