Cell spreading and lamellipodial extension rate is regulated by membrane tension
- PMID: 10629223
- PMCID: PMC2156205
- DOI: 10.1083/jcb.148.1.127
Cell spreading and lamellipodial extension rate is regulated by membrane tension
Abstract
Cell spreading and motility require the extension of the plasma membrane in association with the assembly of actin. In vitro, extension must overcome resistance from tension within the plasma membrane. We report here that the addition of either amphiphilic compounds or fluorescent lipids that expanded the plasma membrane increased the rate of cell spreading and lamellipodial extension, stimulated new lamellipodial extensions, and caused a decrease in the apparent membrane tension. Further, in PDGF-stimulated motility, the increase in the lamellipodial extension rate was associated with a decrease in the apparent membrane tension and decreased membrane-cytoskeleton adhesion through phosphatidylinositol diphosphate hydrolysis. Conversely, when membrane tension was increased by osmotically swelling cells, the extension rate decreased. Therefore, we suggest that the lamellipodial extension process can be activated by a physical signal (perhaps secondarily), and the rate of extension is directly dependent upon the tension in the plasma membrane. Quantitative analysis shows that the lamellipodial extension rate is inversely correlated with the apparent membrane tension. These studies describe a physical chemical mechanism involving changes in membrane-cytoskeleton adhesion through phosphatidylinositol 4,5-biphosphate-protein interactions for modulating and stimulating the biochemical processes that power lamellipodial extension.
Figures





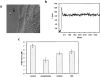
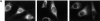
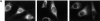

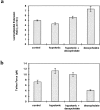


References
-
- Abercrombie M., Heaysman J.E., Pegrum S.M. The locomotion of fibroblasts in culture. 3. Movements of particles on the dorsal surface of the leading lamella. Exp. Cell Res. 1970;62:389–398. - PubMed
-
- Condeelis J. Life at the leading edgethe formation of cell protrusions. Annu. Rev. Cell Biol. 1993;9:411–444. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

