Identification of a new gene locus for adolescent nephronophthisis, on chromosome 3q22 in a large Venezuelan pedigree
- PMID: 10631142
- PMCID: PMC1360127
- DOI: 10.1086/302705
Identification of a new gene locus for adolescent nephronophthisis, on chromosome 3q22 in a large Venezuelan pedigree
Abstract
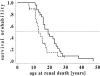
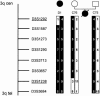
Nephronophthisis, an autosomal-recessive cystic kidney disease, is the most frequent monogenic cause for renal failure in childhood. Infantile and juvenile forms of nephronophthisis are known to originate from separate gene loci. We describe here a new disease form, adolescent nephronophthisis, that is clearly distinct by clinical and genetic findings. In a large, 340-member consanguineous Venezuelan kindred, clinical symptoms and renal pathology were evaluated. Onset of terminal renal failure was compared with that in a historical sample of juvenile nephronophthisis. Onset of terminal renal failure in adolescent nephronophthisis occurred significantly later (median age 19 years, quartile borders 16.0 and 25.0 years) than in juvenile nephronophthisis (median age 13.1 years, quartile borders 11.3 and 17.3 years; Wilcoxon test P=.0069). A total-genome scan of linkage analysis was conducted and evaluated by LOD score and total-genome haplotype analyses. A gene locus for adolescent nephronophthisis was localized to a region of homozygosity by descent, on chromosome 3q22, within a critical genetic interval of 2. 4 cM between flanking markers D3S1292 and D3S1238. The maximum LOD score for D3S1273 was 5.90 (maximum recombination fraction.035). This locus is different than that identified for juvenile nephronophthisis. These findings will have implications for diagnosis and genetic counseling in hereditary chronic renal failure and provide the basis for identification of the responsible gene.
Figures



Similar articles
-
Mapping of a gene for familial juvenile nephronophthisis: refining the map and defining flanking markers on chromosome 2. APN Study Group.Am J Hum Genet. 1993 Dec;53(6):1256-61. Am J Hum Genet. 1993. PMID: 8250041 Free PMC article.
-
Mapping of gene loci for nephronophthisis type 4 and Senior-Løken syndrome, to chromosome 1p36.Am J Hum Genet. 2002 May;70(5):1240-6. doi: 10.1086/340317. Epub 2002 Mar 27. Am J Hum Genet. 2002. PMID: 11920287 Free PMC article.
-
Molecular genetic identification of families with juvenile nephronophthisis type 1: rate of progression to renal failure. APN Study Group. Arbeitsgemeinschaft für Pädiatrische Nephrologie.Kidney Int. 1997 Jan;51(1):261-9. doi: 10.1038/ki.1997.31. Kidney Int. 1997. PMID: 8995741
-
Nephronophthisis: A review of genotype-phenotype correlation.Nephrology (Carlton). 2018 Oct;23(10):904-911. doi: 10.1111/nep.13393. Epub 2018 Jun 21. Nephrology (Carlton). 2018. PMID: 29717526 Free PMC article. Review.
-
New insights: nephronophthisis-medullary cystic kidney disease.Pediatr Nephrol. 2001 Feb;16(2):168-76. doi: 10.1007/s004670000518. Pediatr Nephrol. 2001. PMID: 11261687 Review.
Cited by
-
A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution.Am J Hum Genet. 2002 Nov;71(5):1161-7. doi: 10.1086/344395. Epub 2002 Aug 29. Am J Hum Genet. 2002. PMID: 12205563 Free PMC article.
-
Nephronophthisis: a pathological and genetic perspective.Pediatr Nephrol. 2024 Jul;39(7):1977-2000. doi: 10.1007/s00467-023-06174-8. Epub 2023 Nov 6. Pediatr Nephrol. 2024. PMID: 37930417 Review.
-
Ciliopathies.Cold Spring Harb Perspect Biol. 2017 Mar 1;9(3):a028191. doi: 10.1101/cshperspect.a028191. Cold Spring Harb Perspect Biol. 2017. PMID: 27793968 Free PMC article. Review.
-
Identification of an NPHP1 deletion causing adult form of nephronophthisis.Ir J Med Sci. 2016 Aug;185(3):589-595. doi: 10.1007/s11845-015-1312-7. Epub 2015 Jun 4. Ir J Med Sci. 2016. PMID: 26037636
-
Senior-Løken syndrome: a syndromic form of retinal dystrophy associated with nephronophthisis.Vision Res. 2012 Dec 15;75:88-97. doi: 10.1016/j.visres.2012.07.003. Epub 2012 Jul 20. Vision Res. 2012. PMID: 22819833 Free PMC article. Review.
References
Electronic-Database Information
References
-
- Antignac C, Arduy CH, Beckmann JS, Benessy F, Gros F, Medhioub M, Hildebrandt F, et al (1993) A gene for familial juvenile nephronophthisis (recessive medullary cystic kidney disease) maps to chromosome 2p. Nat Genet 3:342–345 - PubMed
-
- Antignac C, Kleinknecht C, Habib R (1998) Nephronophthisis. In: Cameron J (ed) Textbook of clinical nephrology. Oxford University Press, Oxford, pp 2417–2426
-
- Blowey DL, Querfeld U, Geary D, Warady BA, Alon U (1996) Ultrasound findings in juvenile nephronophthisis. Pediatr Nephrol 10:22–24 - PubMed
-
- Bodaghi E, Honarmand MT, Ahmadi M (1987) Infantile nephronophthisis. Int J Pediatr Nephrol 8:207–210 - PubMed
-
- Boichis H, Passwell J, David R, Miller H (1973) Congenital hepatic fibrosis and nephronophthisis: a family study. Q J Med 42:221–233 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

