Villin-like actin-binding proteins are expressed ubiquitously in Arabidopsis
- PMID: 10631247
- PMCID: PMC58842
- DOI: 10.1104/pp.122.1.35
Villin-like actin-binding proteins are expressed ubiquitously in Arabidopsis
Abstract
In an attempt to elucidate the biological function of villin-like actin-binding proteins in plants we have cloned several genes encoding Arabidopsis proteins with high homology to animal villin. We found that Arabidopsis contains at least four villin-like genes (AtVLNs) encoding four different VLN isoforms. Two AtVLN isoforms are more closely related to mammalian villin in their primary structure and are also antigenically related, whereas the other two contain significant changes in the C-terminal headpiece domain. RNA and promoter/beta-glucuronidase expression studies demonstrated that AtVLN genes are expressed in all organs, with elevated expression levels in certain types of cells. These results suggest that AtVLNs have less-specialized functions than mammalian villin, which is found only in the microvilli of brush border cells. Immunoblot experiments using a monoclonal antibody against pig villin showed that AtVLNs are widely distributed in a variety of plant tissues. Green fluorescent protein fused to full-length AtVLN and individual AtVLN headpiece domains can bind to both animal and plant actin filaments in vivo.
Figures



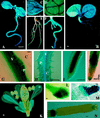

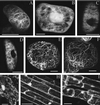
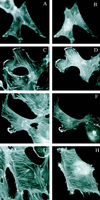
References
-
- Andre E, Lottspeich F, Schleicher M, Noegel A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem. 1988;263:722–727. - PubMed
-
- Aoyama T, Chua N-H. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612. - PubMed
-
- Arpin M, Pringault E, Finidori J, Garcia A, Jeltsch J-M, Vandekerckhove J, Louvard D. Sequence of human villin: a large duplicated domain homologous with other actin-severing proteins and a unique small carboxy-terminal domain related to villin specificity. J Cell Biol. 1988;107:1759–1766. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

