Passive nitrate transport by root plasma membrane vesicles exhibits an acidic optimal pH like the H(+)-ATPase
- PMID: 10631270
- PMCID: PMC58865
- DOI: 10.1104/pp.122.1.265
Passive nitrate transport by root plasma membrane vesicles exhibits an acidic optimal pH like the H(+)-ATPase
Abstract
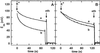
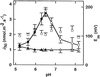
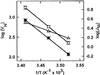
The net initial passive flux (J(Ni)) in reconstituted plasma membrane (PM) vesicles from maize (Zea mays) root cells was measured as recently described (P. Pouliquin, J.-P. Grouzis, R. Gibrat ¿1999 Biophys J 76: 360-373). J(Ni) in control liposomes responded to membrane potential or to NO(3)(-) as expected from the Goldman-Hodgkin-Katz diffusion theory. J(Ni) in reconstituted PM vesicles exhibited an additional component (J(Nif)), which was saturable (K(m) for NO(3)(-) approximately 3 mM, with J(Nifmax) corresponding to 60 x 10(-9) mol m(-2) s(-1) at the native PM level) and selective (NO(3)(-) = ClO(3)(-) > Br(-) > Cl(-) = NO(2)(-); relative fluxes at 5 mM: 1:0.34:0.19). J(Nif) was totally inhibited by La(3+) and the arginine reagent phenylglyoxal. J(Nif) was voltage dependent, with an optimum voltage at 105 mV at pH 6.5. The activation energy of J(Nif) was high (129 kJ mol(-1)), close to that of the H(+)-ATPase (155 kJ mol(-1)), and J(Nif) displayed the same acidic optimal pH (pH 6.5) as that of the H(+) pump. This is the first example, to our knowledge, of a secondary transport at the plant PM with such a feature. Several properties of the NO(3)(-) uniport seem poorly compatible with that reported for plant anion channels and to be attributable instead to a classical carrier. The physiological relevance of these findings is suggested.
Figures


Similar articles
-
In vitro study of passive nitrate transport by native and reconstituted plasma membrane vesicles from corn root cells.Biochim Biophys Acta. 1997 Apr 26;1325(2):329-42. doi: 10.1016/s0005-2736(96)00256-8. Biochim Biophys Acta. 1997. PMID: 9168158
-
Electrophysiological study with oxonol VI of passive NO3- transport by isolated plant root plasma membrane.Biophys J. 1999 Jan;76(1 Pt 1):360-73. doi: 10.1016/s0006-3495(99)77203-6. Biophys J. 1999. PMID: 9876148 Free PMC article.
-
Coumarin enhances nitrate uptake in maize roots through modulation of plasma membrane H+ -ATPase activity.Plant Biol (Stuttg). 2018 Mar;20(2):390-398. doi: 10.1111/plb.12674. Epub 2017 Dec 21. Plant Biol (Stuttg). 2018. PMID: 29181876
-
Characterization of a H+/NO3- symport associated with plasma membrane vesicles of maize roots using 36ClO3- as a radiotracer analog.Arch Biochem Biophys. 1991 Feb 15;285(1):74-82. doi: 10.1016/0003-9861(91)90330-l. Arch Biochem Biophys. 1991. PMID: 1990981
-
Nitrate uptake along the maize primary root: an integrated physiological and molecular approach.Plant Cell Environ. 2011 Jul;34(7):1127-40. doi: 10.1111/j.1365-3040.2011.02311.x. Epub 2011 Apr 21. Plant Cell Environ. 2011. PMID: 21410710
Cited by
-
pOsNAR2.1:OsNAR2.1 expression enhances nitrogen uptake efficiency and grain yield in transgenic rice plants.Plant Biotechnol J. 2017 Oct;15(10):1273-1283. doi: 10.1111/pbi.12714. Epub 2017 Mar 29. Plant Biotechnol J. 2017. PMID: 28226420 Free PMC article.
-
Plant nitrogen uptake and assimilation: regulation of cellular pH homeostasis.J Exp Bot. 2020 Jul 25;71(15):4380-4392. doi: 10.1093/jxb/eraa150. J Exp Bot. 2020. PMID: 32206788 Free PMC article. Review.
-
Uptake of sulfate but not phosphate by Mycobacterium tuberculosis is slower than that for Mycobacterium smegmatis.J Bacteriol. 2012 Mar;194(5):956-64. doi: 10.1128/JB.06132-11. Epub 2011 Dec 22. J Bacteriol. 2012. PMID: 22194452 Free PMC article.
-
Nitrate efflux at the root plasma membrane: identification of an Arabidopsis excretion transporter.Plant Cell. 2007 Nov;19(11):3760-77. doi: 10.1105/tpc.106.048173. Epub 2007 Nov 9. Plant Cell. 2007. PMID: 17993627 Free PMC article.
References
-
- Alam SM. Nutrient uptake by plants under stress conditions. In: Pessarakali M, editor. Handbook of Plant Crop Stress. New York: Marcel Dekker; 1994. pp. 227–246.
-
- Amancio S, Diogo E, Santos H. Effects of the source of inorganic nitrogen on C and N interaction in maize callus tissue: phosphoenolpyruvate carboxylase activity, cytoplasmic pH and 15N amino acids. Physiol Plant. 1993;89:618–625.
-
- Appel HJ, Häring V, Rounda M. Na,K-ATPase in artificial lipid vesicles: comparison of Na, K and Na-only pumping mode. Biochim Biophys Acta. 1990;1023:81–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

