Nerve injury induces gap junctional coupling among axotomized adult motor neurons
- PMID: 10632597
- PMCID: PMC6772393
- DOI: 10.1523/JNEUROSCI.20-02-00674.2000
Nerve injury induces gap junctional coupling among axotomized adult motor neurons
Abstract
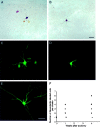
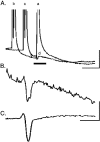
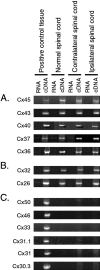
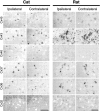
Neonatal spinal motor neurons are electrically and dye-coupled by gap junctions, but coupling is transient and disappears rapidly after birth. Here we report that adult motor neurons become recoupled by gap junctions after peripheral nerve injury. One and 4-6 weeks after nerve cut, clusters of dye-coupled motor neurons were observed among axotomized, but not control, lumbar spinal motor neurons in adult cats. Electrical coupling was not apparent, probably because of the electrotonic distance between dendrodendritic gap junctions and the somatic recording location. Analyses of gap junction protein expression in cat and rat showed that the repertoire of connexins expressed by normal adult motor neurons, Cx36, Cx37, Cx40, Cx43, and Cx45, was unchanged after axotomy. Our results suggest that the reestablishment of gap junctional coupling among axotomized adult motor neurons may occur by modulation of existing gap junction proteins that are constitutively expressed by motor neurons. After injury, interneuronal gap junctional coupling may mediate signaling that maintains the viability of axotomized motor neurons until synaptic connections are reestablished within their targets.
Figures

References
-
- Aldskogius H, Kozlova EN. Central neuron–glial and glial–glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. - PubMed
-
- Balice-Gordon RJ, Pereda A, Pinter MJ. Functional gap junctions couple motor neurons in development and reinnervation. Soc Neurosci Abstr. 1996;22:1487.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
