Relationship of activity in the subthalamic nucleus-globus pallidus network to cortical electroencephalogram
- PMID: 10632612
- PMCID: PMC6772398
- DOI: 10.1523/JNEUROSCI.20-02-00820.2000
Relationship of activity in the subthalamic nucleus-globus pallidus network to cortical electroencephalogram
Abstract
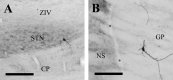
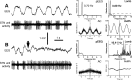
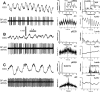
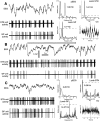
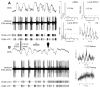
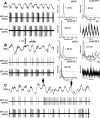
One of the functions of the excitatory subthalamic nucleus (STN) is to relay cortical activity to other basal ganglia structures. The response of the STN to cortical input is shaped by inhibition from the reciprocally connected globus pallidus (GP). To examine the activity in the STN-GP network in relation to cortical activity, we recorded single and multiple unit activity in STN and/or GP together with cortical electroencephalogram in anesthetized rats during various states of cortical activation. During cortical slow-wave activity (SWA), STN and GP neurons fired bursts of action potentials at frequencies that were similar to those of coincident slow ( approximately 1 Hz) and spindle (7-14 Hz) cortical oscillations. Spontaneous or sensory-driven global activation was associated with a reduction of SWA and a shift in STN-GP activity from burst- to tonic- or irregular-firing. Rhythmic activity in STN and GP neurons was lost when the cortex was inactivated by spreading depression and did not resume until SWA had recovered. Although rhythmic STN-GP activity was correlated with SWA, the phase relationships of activities of neurons within the STN and GP and between the nuclei were variable. Even when neurons displayed synchronous bursting activity, correlations on the millisecond time scale, which might indicate shared synaptic input, were not observed. These data indicate that (1) STN and GP activity is intimately related to cortical activity and hence the sleep-wake cycle; (2) rhythmic oscillatory activity in the STN-GP network in disease states may be driven by the cortex; and (3) activity of the STN-GP network is regulated in space in a complex manner.
Figures

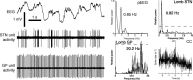
Similar articles
-
Unrelated course of subthalamic nucleus and globus pallidus neuronal activities across vigilance states in the rat.Eur J Neurosci. 2000 Sep;12(9):3361-74. doi: 10.1046/j.1460-9568.2000.00199.x. Eur J Neurosci. 2000. PMID: 10998119
-
Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network.Neuroscience. 2001;106(2):313-30. doi: 10.1016/s0306-4522(01)00281-0. Neuroscience. 2001. PMID: 11566503
-
Functional interconnectivity between the globus pallidus and the subthalamic nucleus in the mouse brain slice.J Physiol. 2005 Sep 15;567(Pt 3):977-87. doi: 10.1113/jphysiol.2005.093807. Epub 2005 Jul 21. J Physiol. 2005. PMID: 16037086 Free PMC article.
-
Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network.Trends Neurosci. 2002 Oct;25(10):525-31. doi: 10.1016/s0166-2236(02)02235-x. Trends Neurosci. 2002. PMID: 12220881 Review.
-
GABAergic control of the subthalamic nucleus.Prog Brain Res. 2007;160:173-88. doi: 10.1016/S0079-6123(06)60010-1. Prog Brain Res. 2007. PMID: 17499114 Review.
Cited by
-
Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models.Materials (Basel). 2019 Oct 1;12(19):3218. doi: 10.3390/ma12193218. Materials (Basel). 2019. PMID: 31581436 Free PMC article. Review.
-
Synchronized neuronal discharge in the basal ganglia of parkinsonian patients is limited to oscillatory activity.J Neurosci. 2002 Apr 1;22(7):2855-61. doi: 10.1523/JNEUROSCI.22-07-02855.2002. J Neurosci. 2002. PMID: 11923450 Free PMC article. Clinical Trial.
-
Synaptic Changes in Pallidostriatal Circuits Observed in the Parkinsonian Model Triggers Abnormal Beta Synchrony with Accurate Spatio-temporal Properties across the Basal Ganglia.J Neurosci. 2024 Feb 28;44(9):e0419232023. doi: 10.1523/JNEUROSCI.0419-23.2023. J Neurosci. 2024. PMID: 38123981 Free PMC article.
-
Interaction of slow cortical rhythm with somatosensory information processing in urethane-anesthetized rats.Brain Res. 2008 Aug 21;1226:99-110. doi: 10.1016/j.brainres.2008.05.068. Epub 2008 Jun 5. Brain Res. 2008. PMID: 18588861 Free PMC article.
-
Nav1.6 sodium channels are critical to pacemaking and fast spiking in globus pallidus neurons.J Neurosci. 2007 Dec 5;27(49):13552-66. doi: 10.1523/JNEUROSCI.3430-07.2007. J Neurosci. 2007. PMID: 18057213 Free PMC article.
References
-
- Abeles M. Quantification, smoothing, and confidence limits for single-units' histograms. J Neurosci Methods. 1982;5:317–325. - PubMed
-
- Achermann P, Borbély AA. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. - PubMed
-
- Albe-Fessard D, Condes-Lara M, Kesar S, Sanderson P. Tonic cortical controls acting on spontaneous and evoked thalamic activity. In: Macchi G, Rustioni A, Spreafico R, editors. Somatosensory integration in the thalamus. Elsevier; Amsterdam: 1983. pp. 273–285.
-
- Aldridge JW, Gilman S. The temporal structure of spike trains in the primate basal ganglia: afferent regulation of bursting demonstrated with precentral cerebral cortical ablation. Brain Res. 1991;543:123–138. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
