Does informed consent alter elderly patients' preferences for colorectal cancer screening? Results of a randomized trial
- PMID: 10632830
- PMCID: PMC1495322
- DOI: 10.1046/j.1525-1497.2000.01079.x
Does informed consent alter elderly patients' preferences for colorectal cancer screening? Results of a randomized trial
Abstract
Objective: To assess the impact of informed consent on elderly patients' colorectal cancer (CRC) screening preferences.
Design: Randomized, controlled trial.
Setting: Four general internal medicine practices.
Patients: We studied 399 elderly patients visiting their primary care provider for routine office visits.
Interventions: Patients were randomized to receive either a scripted control message briefly describing CRC screening methods or one of two informational interventions simulating an informed consent presentation about CRC screening. One intervention described CRC mortality risk reduction in relative terms; the other, in absolute terms.
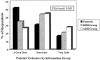
Measurements and main results: The main outcome measure was intent to begin or continue fecal occult blood testing (FOBT), flexible sigmoidoscopy, or both. There was no difference in screening interest between the control group and the two information groups (p =.8). The majority (63%) of patients intended to begin or continue CRC screening. Informed patients were able to gauge more accurately the positive predictive value of screening (p =.0009). Control patients rated the efficacy of screening higher than did patients receiving relative risk reduction information, who rated it higher than did patients receiving absolute risk reduction information (p =.0002).
Conclusions: Elderly patients appeared to understand CRC screening information and use it to gauge the efficacy of screening, but provision of information had no impact on their preferences for screening. In view of the large proportion who preferred not to be screened, we conclude that elderly patients should be involved in the screening decision. However, factors other than provision of information must determine their CRC screening preferences.
Figures



References
-
- Landis SH, Murray T, Bolden S. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. - PubMed
-
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328:1365–71. - PubMed
-
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. - PubMed
-
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–77. - PubMed
-
- Selby JV, Friedman GD, Quesenberry CP, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
