Succinate:quinol oxidoreductases in the cyanobacterium synechocystis sp. strain PCC 6803: presence and function in metabolism and electron transport
- PMID: 10633105
- PMCID: PMC94334
- DOI: 10.1128/JB.182.3.714-722.2000
Succinate:quinol oxidoreductases in the cyanobacterium synechocystis sp. strain PCC 6803: presence and function in metabolism and electron transport
Abstract
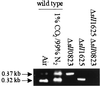
The open reading frames sll1625 and sll0823, which have significant sequence similarity to genes coding for the FeS subunits of succinate dehydrogenase and fumarate reductase, were deleted singly and in combination in the cyanobacterium Synechocystis sp. strain PCC 6803. When the organic acid content in the Deltasll1625 and Deltasll0823 strains was analyzed, a 100-fold decrease in succinate and fumarate concentrations was observed relative to the wild type. A similar analysis for the Deltasll1625 Deltasll0823 strain revealed that 17% of the wild-type succinate levels remained, while only 1 to 2% of the wild-type fumarate levels were present. Addition of 2-oxoglutarate to the growth media of the double mutant strain prior to analysis of organic acids in cells caused succinate to accumulate. This indicates that succinate dehydrogenase activity had been blocked by the deletions and that 2-oxoglutarate can be converted to succinate in vivo in this organism, even though a traditional 2-oxoglutarate dehydrogenase is lacking. In addition, reduction of the thylakoid plastoquinone pool in darkness in the presence of KCN was up to fivefold slower in the mutants than in the wild type. Moreover, in vitro succinate dehydrogenase activity observed in wild-type membranes is absent from those isolated from the double mutant and reduced in those from the single mutants, further indicating that the sll1625 and sll0823 open reading frames encode subunits of succinate dehydrogenase complexes that are active in the thylakoid membrane of the cyanobacterium.
Figures




References
-
- Ackrell B A C, Armstrong F A, Cochran B, Sucheta A, Yu T. Classification of fumarate reductases and succinate dehydrogenases based upon their contrasting behavior in the reduced benzylviologen fumarate assay. FEBS Lett. 1993;326:92–94. - PubMed
-
- Bendall D S, Manasse R S. Cyclic photophosphorylation and electron transport. Biochim Biophys Acta. 1995;1229:23–38.
-
- Berger S, Ellersiek U, Steinmüller K. Cyanobacteria contain a mitochondrial complex-I homologous NADH-dehydrogenase. FEBS Lett. 1991;286:129–132. - PubMed
-
- Binder A, Hauser R, Krogmann D. Respiration in energy-transducing membranes of the thermophilic cyanobacterium Mastigocladus laminosus. Biochim Biophys Acta. 1984;765:241–246.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

