On the functional interchangeability, oxidant versus reductant, of members of the thioredoxin superfamily
- PMID: 10633106
- PMCID: PMC94335
- DOI: 10.1128/JB.182.3.723-727.2000
On the functional interchangeability, oxidant versus reductant, of members of the thioredoxin superfamily
Abstract
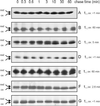
Escherichia coli thioredoxin 1 has been characterized in vivo and in vitro as one of the most efficient reductants of disulfide bonds. Nevertheless, under some conditions, thioredoxin 1 can also act in vivo as an oxidant, promoting formation of disulfide bonds in the cytoplasm (E. J. Stewart, F. Aslund, and J. Beckwith, EMBO J. 17:5543-5550, 1998). We recently showed that when a signal sequence is attached to thioredoxin 1 it is exported to the periplasm, where it can also act as an oxidant, replacing the normal periplasmic catalyst of disulfide bond formation, DsbA, in oxidizing cell envelope proteins (L. Debarbieux and J. Beckwith, Proc. Natl. Acad. Sci. USA 95:10751-10756, 1998). Here we report pulse-chase studies of the efficiency of disulfide bond formation in strains exporting thioredoxin 1 and more-oxidizing variants of it. While the exported thioredoxin 1 itself substantially speeds up the kinetics of disulfide bond formation, a version of this protein containing the DsbA active site exhibits kinetics that are indistinguishable from those of the DsbA protein itself. Further, we confirm the findings of Jonda et al. (S. Jonda, M. Huber-Wunderlich, R. Glockshuber, and E. Mössner, EMBO J. 18:3271-3281, 1999), who found that DsbB is responsible for the oxidation of exported thioredoxin 1, and we report the detection of a disulfide-bonded DsbB-thioredoxin 1 complex. Finally, we have found that under conditions of high-level expression of exported thioredoxin 1, the protein can act as both an oxidant and a reductant.
Figures
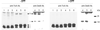
References
-
- Åslund F, Berndt K D, Holmgren A. Redox potentials of glutaredoxins and other thiol-disulfide oxidoreductases of the thioredoxin superfamily determined by direct protein-protein redox equilibria. J Biol Chem. 1997;272:30780–30786. - PubMed
-
- Bader M, Muse W, Ballou D P, Gassner C, Bardwell J C. Oxidative protein folding is driven by the electron transport system. Cell. 1999;98:217–227. - PubMed
-
- Bardwell J C A, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. - PubMed
-
- Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and φ80 transducing phages. J Mol Biol. 1975;96:307–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

