Posttranslational processing of Methanococcus voltae preflagellin by preflagellin peptidases of M. voltae and other methanogens
- PMID: 10633127
- PMCID: PMC94356
- DOI: 10.1128/JB.182.3.855-858.2000
Posttranslational processing of Methanococcus voltae preflagellin by preflagellin peptidases of M. voltae and other methanogens
Abstract
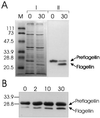
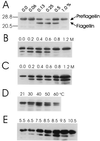
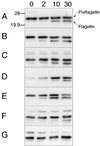
Methanococcus voltae is a mesophilic archaeon with flagella composed of flagellins that are initially made with 11- or 12-amino-acid leader peptides that are cleaved prior to incorporation of the flagellin into the growing filament. Preflagellin peptidase activity was demonstrated in immunoblotting experiments with flagellin antibody to detect unprocessed and processed flagellin subunits. Escherichia coli membranes containing the expressed M. voltae preflagellin (as the substrate) were combined in vitro with methanogen membranes (as the enzyme source). Correct processing of the preflagellin to the mature flagellin was also shown directly by comparison of the N-terminal sequences of the two flagellin species. M. voltae preflagellin peptidase activity was optimal at 37 degrees C and pH 8.5 and in the presence of 0.4 M KCl with 0.25% (vol/vol) Triton X-100.
Figures

References
-
- Albers S-V, Konings W N, Driessen A J M. A unique short signal sequence in membrane-anchored proteins of Archaea. Mol Microbiol. 1999;31:1595–1596. - PubMed
-
- Bayley D P, Florian V, Klein A, Jarrell K F. Flagellin genes of Methanococcus vannielii: amplification by the polymerase chain reaction, demonstration of signal peptides and identification of major components of the flagellar filament. Mol Gen Genet. 1998;258:639–645. - PubMed
-
- Bayley D P, Jarrell K F. Further evidence to suggest that archaeal flagella are related to bacterial type IV pili. J Mol Evol. 1998;46:370–373. - PubMed
-
- Bröckl G, Behr M, Farby S, Hensel R, Kaudewitz H, Biendel E, König H. Analysis and nucleotide sequence of the genes encoding the surface-layer glycoproteins of the hyperthermophilic methanogens Methanothermus fervidus and Methanothermus sociabilis. Eur J Biochem. 1991;199:147–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

