Far upstream regulatory elements enhance position-independent and uterus-specific expression of the murine alpha1(I) collagen promoter in transgenic mice
- PMID: 10634317
- PMCID: PMC6157370
Far upstream regulatory elements enhance position-independent and uterus-specific expression of the murine alpha1(I) collagen promoter in transgenic mice
Abstract
The stage- and tissue-specific expression of many eukaryotic genes is regulated by cis-regulatory elements, some of which are located in proximity to the start site of transcription whereas others have been identified at considerable distances. In previous studies we have identified far upstream DNase I-hypersensitive sites in the murine alpha1(I) collagen (Col1a1) gene, which may play a role in the regulation of this abundantly expressed gene. Here we have cloned several of these sites into reporter gene constructs containing the Col1a1 promoter driving the green fluorescent protein (GFP) reporter gene and tested their possible functions in transfection experiments and transgenic mice. In transient and stable transfections none of the hypersensitive sites had a significant effect on Col1a1 promoter activity, indicating that they do not contain a classical transcriptional enhancer. In transgenic animals one element located at -18 to -19.5 kb enhanced the position-independent activity of the linked Col1a1 promoter and may be part of a locus control region. Another element located at -7 to -8 kb specifically enhanced reporter gene expression in the uteri of transgenic mice, suggesting that it contains a novel transcriptional enhancer that may be involved in the regulation of type I collagen expression in tissue remodeling in the uterus during the estrous cycle. Our studies also demonstrate the versatility of the GFP reporter gene for use in transgenic animals because it can be analyzed in live animals, whole mount embryos, histological thin sections, or primary cell cultures, and it can be quantified very sensitively in tissue or cell extracts using a fluorometer.
Figures
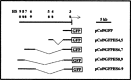

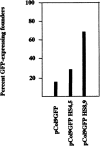
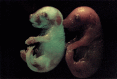
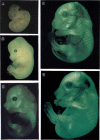
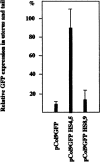
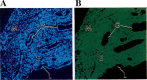
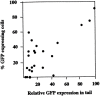
References
-
- Barsh G. S.; Roush C. L.; Gelinas R. E. DNA and chromatin structure of the human alpha1 (I) collagen gene. J. Biol. Chem. 259:14906–14913; 1984. - PubMed
-
- Bedalov A.; Salvatori R.; Dodig M.; Kronenberg M. S.; Kapural B.; Bogdanovic Z.; Kream B. E.; Woody C. O.; Clark S. H.; Mack K.; Rowe D. W.; Lichtler A. L. Regulation of COL1A1 gene expression in type I collagen producing tissues: Identification of a 49 base pair region which is required for transgene expression in bone of transgenic mice. J. Bone Miner. Res. 10:1443–1452; 1995. - PubMed
-
- Bogdanovic Z.; Bedalov A.; Krebsbach P. H.; Pavlin D.; Woody C. O.; Clark S. H.; Thomas H. F.; Rowe D. W.; Kream B. E.; Lichtler A. C. Upstream regulatory elements necessary for expression of the rat COL1A1 promoter in transgenic mice. J. Bone Miner. Res. 9:285–292; 1994. - PubMed
-
- Bornstein P. Regulation of expression of the al (I) collagen gene: A critical appraisal of the role of the first intron. Matrix Biol. 15:3–10; 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
