Monoubiquitin carries a novel internalization signal that is appended to activated receptors
- PMID: 10637223
- PMCID: PMC305553
- DOI: 10.1093/emboj/19.2.187
Monoubiquitin carries a novel internalization signal that is appended to activated receptors
Abstract
Ubiquitin modification of signal transducing receptors at the plasma membrane is necessary for rapid receptor internalization and downregulation. We have investigated whether ubiquitylation alters a receptor cytoplasmic tail to reveal a previously masked internalization signal, or whether ubiquitin itself carries an internalization signal. Using an alpha-factor receptor-ubiquitin chimeric protein, we demonstrate that monoubiquitin can mediate internalization of an activated receptor that lacks all cytoplasmic tail sequences. Furthermore, fusion of ubiquitin in-frame to the stable plasma membrane protein Pma1p stimulates endocytosis of this protein. Ubiquitin does not carry a functional tyrosine- or di-leucine-based internalization signal. Instead, the three-dimensional structure of the folded ubiquitin polypeptide carries an internalization signal that consists of two surface patches surrounding the critical residues Phe4 and Ile44. We conclude that ubiquitin functions as a novel regulated internalization signal that can be appended to a plasma membrane protein to trigger downregulation.
Figures


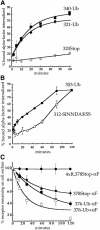
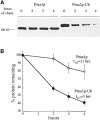
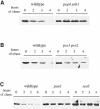

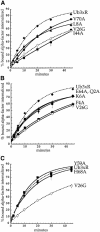

References
-
- Benito B., Moreno, E. and Lagunas, R. (1991) Half-life of the plasma membrane ATPase and its activating system in resting yeast cells. Biochim. Biophys. Acta, 1063, 265–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

