CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo
- PMID: 10637271
- PMCID: PMC2195756
- DOI: 10.1084/jem.191.2.265
CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo
Abstract
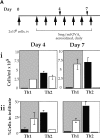
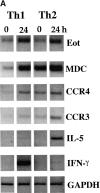
Isolated peripheral blood CD4 cells from allergic individuals express CC chemokine receptor (CCR)3 and CCR4 after expansion in vitro. In addition, human T helper type 2 (Th2) cells polarized in vitro selectively express CCR3 and CCR4 at certain stages of activation/differentiation and respond preferentially to the ligands eotaxin and monocyte-derived chemokine (MDC). However, controversy arises when the in vivo significance of this distinct expression is discussed. To address the functional role of CCR3/eotaxin and CCR4/MDC during the in vivo recruitment of Th2 cells, we have transferred effector Th cells into naive mice to induce allergic airway disease. Tracking of these cells after repeated antigen challenge has established that both CCR3/eotaxin and CCR4/MDC axes contribute to the recruitment of Th2 cells to the lung, demonstrating the in vivo relevance of the expression of these receptors on Th2 cells. We have shown that involvement of the CCR3/eotaxin pathway is confined to early stages of the response in vivo, whereas repeated antigen stimulation results in the predominant use of the CCR4/MDC pathway. We propose that effector Th2 cells respond to both CCR3/eotaxin and CCR4/MDC pathways initially, but that a progressive increase in CCR4-positive cells results in the predominance of the CCR4/MDC axis in the long-term recruitment of Th2 cells in vivo.
Figures









References
-
- Mossmann T.R., Coffman R.L. TH1 and TH2 cellsdifferent patterns of cytokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. - PubMed
-
- Sallusto F., Mackay C.R., Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

